PD-L1 targeted sorafenib-loaded PLGA nano preparation and preparation method thereof
A PD-L1, nano-formulation technology, applied in the field of biomedicine, can solve the problem of not being able to further promote the absorption of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of PD-L1 Targeted Loaded Sorafenib PLGA Nano-Preparation (referred to as PDL1-SRF-PLGA NPs)
[0043] 1. Weigh Sorafenib (SRF), dissolve in DMSO, and configure a concentration of 20mg / ml, weigh PLGA, dissolve in dichloromethane, and configure a concentration of 20mg / mL, vortex mix the SRF solution and the PLGA solution (volume Ratio 1:20), to obtain the organic phase.
[0044] 2. Weigh PVA and dissolve it in deionized water to obtain an aqueous phase with a concentration of 10 mg / ml.
[0045] 3. Take the organic phase with a pipette gun, quickly add it to the water phase (the volume ratio of the organic phase to the water phase is 1:15), ultrasonicate for 15 minutes to obtain an emulsion, stir with a magnetic stirrer for 4 hours, remove the organic phase, and the carrier material is precipitated Solidification forms nanoparticles.
[0046] 4. Centrifuge at 3000rpm to remove large-particle nano-preparation and unencapsulated Sorafenib to obtain Sor...
Embodiment 2
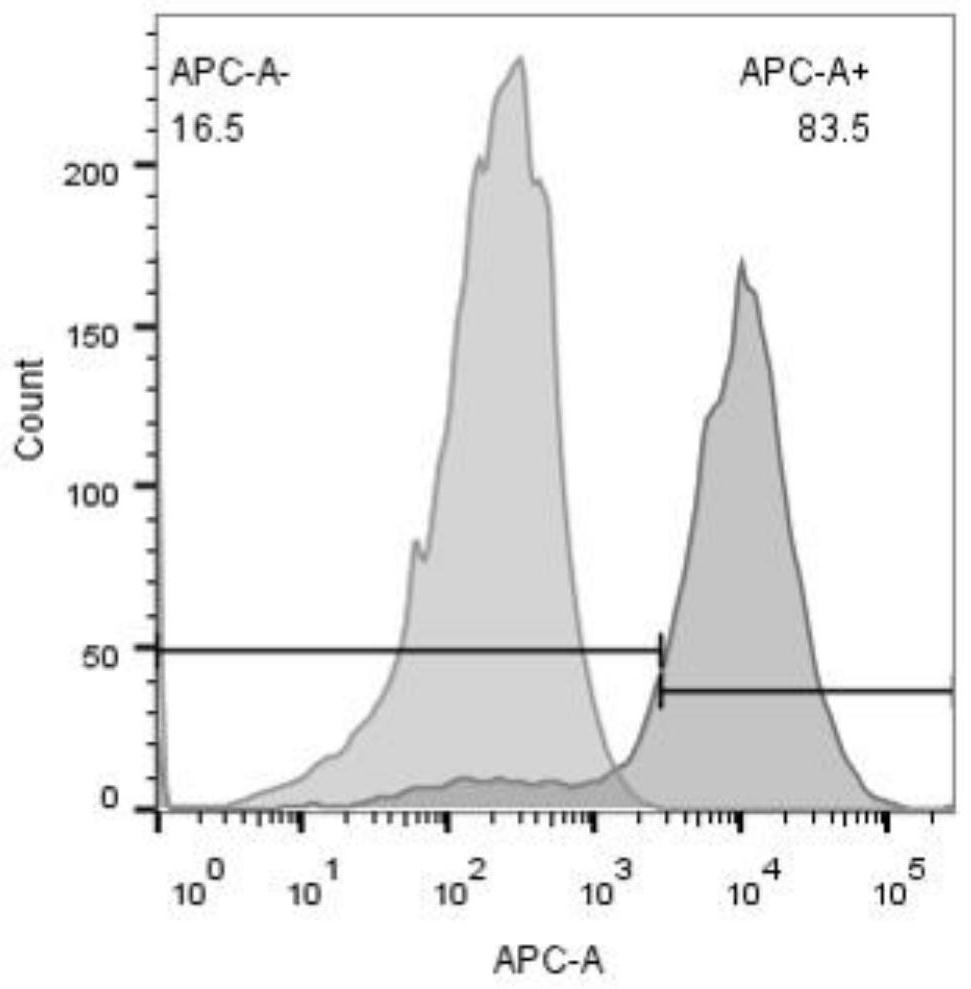
[0061] Example 2 Expression of PD-L1 in the tumor microenvironment of the human liver cancer cell line BEL-7402
[0062] 1. Dissect the BEL-7402 tumor-bearing mice, take out the tumor mass, remove blood clots, mucous membranes and vacuolar necrotic tissue, cut into small pieces, place them in a 70 μm sieve, dilute with 5% FBS-PBS, and place on ice Gently grind and sieve to make a single cell suspension.
[0063] 2. Collect the single-cell suspension in a sterile centrifuge tube, centrifuge at 500g at 4°C for 10 minutes, discard the upper turbid solution, resuspend the cell pellet with diluent, and wash twice by centrifugation;
[0064] 3. Resuspend the cell pellet in 100 μl of diluent, add 0.5 μl of rabbit anti-human PD-L1 antibody, mix well, and incubate at 4°C for 1 hour.
[0065] 4. Centrifuge and wash twice.
[0066] 5. Resuspend the cell pellet in 100 μl of diluent, add 0.5 μl of fluorescently labeled goat anti-rabbit secondary antibody, mix well, and incubate at 4°C fo...
Embodiment 3
[0069] Example 3 Performance testing of PD-L1 targeting loaded Sorafenib PLGA nano-preparation
[0070] The medicine was prepared with reference to Example 1.
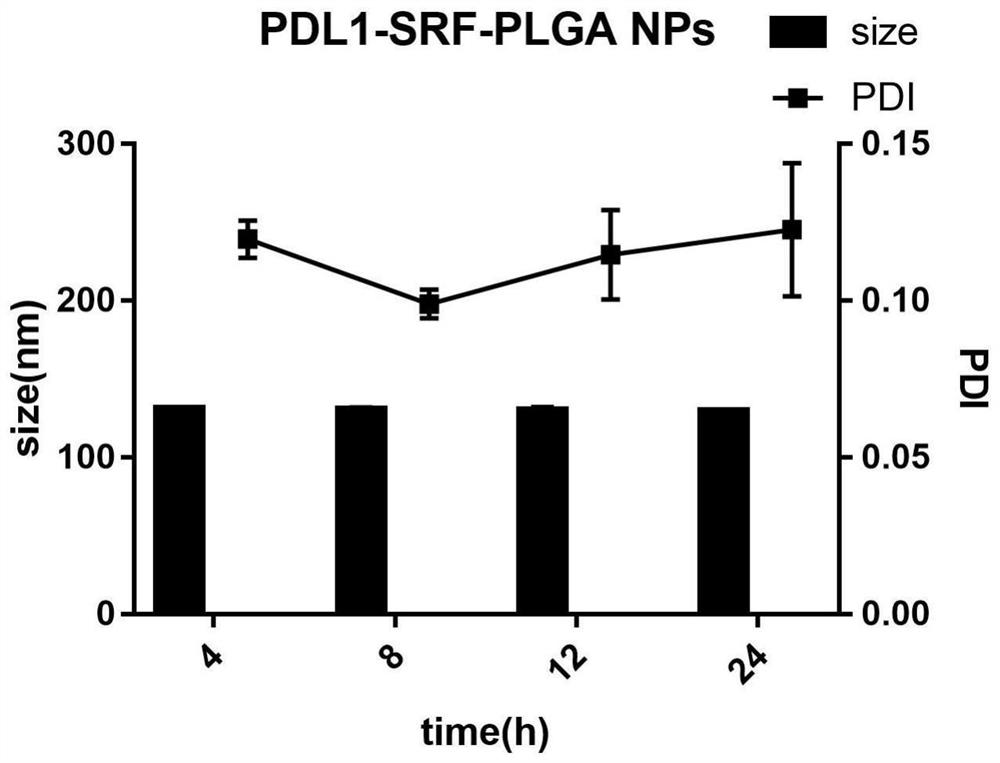
[0071] 1. The particle size and polydispersity coefficient of PDL1-SRF-PLGA NPs were measured by laser particle size analyzer.
[0072] The results showed that the size of PDL1-SRF-PLGA NPs was 145.4±1.6nm, suitable for gathering in the tumor; the polydispersity coefficient was 0.120±0.006, and the distribution was relatively uniform.
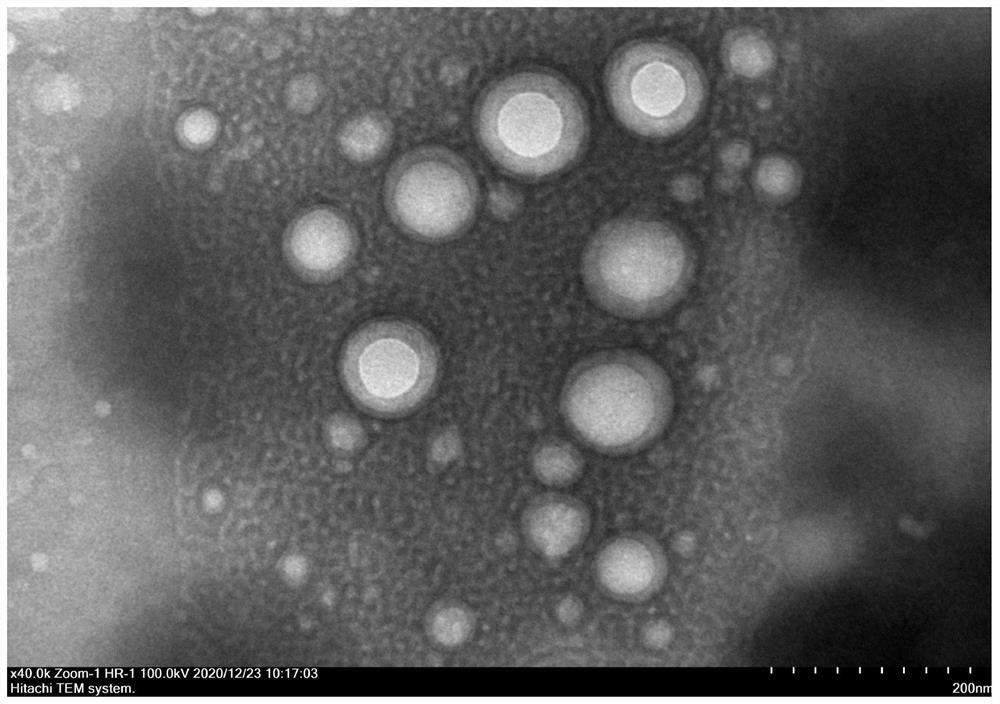
[0073] 2. The morphology of PDL1-SRF-PLGA NPs was observed by counterstaining with transmission electron microscope.
[0074] Such as figure 2 As shown, the transmission electron microscope (TEM) image shows that the PDL1-SRF-PLGA NPs have a clear spherical structure, which is consistent with the particle size determined by the laser particle size analyzer.
[0075] 3. The encapsulation efficiency and drug loading of PDL1-SRF-PLGA NPs were detected by HPLC.
[0076] The encapsulati...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap