Calcium ion fluorescence probe based on aggregation induced effect, preparation method and uses thereof
A calcium ion and synthesis method technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of few reports on fluorescent probes, achieve wide detection concentration range, wide applicable pH range, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0029] Example 1: Synthesis of probe
[0030] In order to understand the present invention more clearly, the following further describes the present invention in detail through specific embodiments. 4-Hydroxybenzophenone (1.98 g, 10 mmol) and potassium carbonate (4.14 g, 30 mmol) were added to 50 mL of acetone solution, followed by slowly adding 1-bromo-2-chloroethane (1.43 g) at room temperature , 10 mmol). The mixture was heated to reflux for 8 h, and then filtered. The filtrate was concentrated and recrystallized from methanol to obtain compound 2 as a white solid with a yield of 70%.
[0031] 1 H NMR (400 MHz, CDCl 3 ), δ (ppm): 7.83 (d, J = 8.7Hz, 2H); 7.76 (d, J = 7.5Hz, 2H); 7.58 (d, 1H); 7.47 (m, J = 6.9Hz, 2H); 6.98 (d, J = 6.8Hz, 2H); 4.38 (t, J = 7.5Hz, 2H); 3.69 (t, J = 7.5Hz, 2H). 13 C NMR (100 MHz, CDCl 3 ), δ(ppm): 195.86, 162.21, 139.46, 133.07, 132.48, 130.18, 128.91, 128.63,114.46, 68.42, 29.05. ESI-MS: m / z calc’d for C 15 H 13 ClO 2 : [M+Na] + 326.9996;fo
Example Embodiment
[0038] Example 2: Determination of ion selectivity by probe
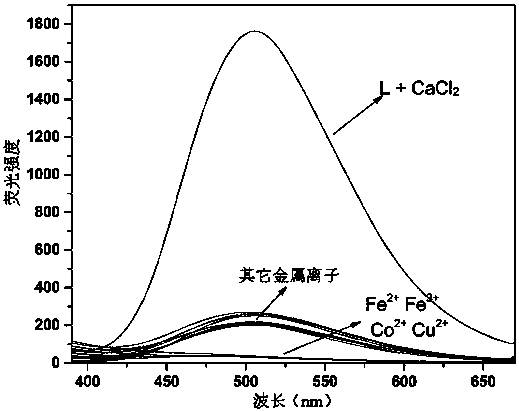
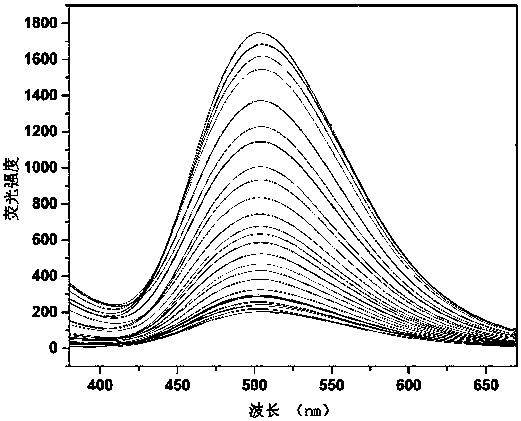
[0039] Use DMSO / HEPES buffer (10 mM, 1:99 v / v) as the solvent to accurately configure the probe with a concentration of 20 μM, respectively mix the solution with 5 times the equivalent of various metal ions, and measure its excitation wavelength at 340 nm Fluorescence emission spectrum under the measurement result as figure 1 Shown. It can be seen that the probe is only for Ca 2+ Shows obvious selective response, no obvious response to other ions, especially to Mg 2+ There is no obvious response signal.
Example Embodiment
[0040] Example 3: Determination of the anti-interference ability of the probe
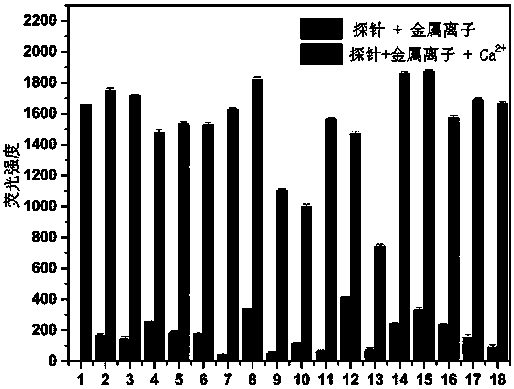
[0041] Use DMSO / HEPES buffer (10 mM, 1:99 v / v) as the solvent to accurately configure the concentration of the 20 μM probe solution, and first mix with 5 times the equivalent of Ca 2+ Mix the solution with 5 times the equivalent of various interfering ions, and measure the fluorescence emission spectrum at the excitation wavelength of 490 nm. The measurement results are as follows figure 2 . Of which 1: Ca 2+ ; 2: Na + ; 3: K + ; 4: Ag + ; 5:Mg 2+ ; 6: Al 3+ ; 7: Cr 3+ ; 8: Mn 2+ ; 9: Fe 2+ ; 10: Fe 3+ ; 11: Co 2+ ; 12: Ni 2+ ; 13: Cu 2+ ;14: Zn 2+ ; 15: Cd 2+ ; 16: Ba 2+ ; 17: Hg 2+ ; 18: Pb 2+ . It can be seen that in addition to Cu 2+ , Co 2+ , Fe 2+ And Fe 3+ In addition to a certain degree of fluorescence quenching due to its paramagnetism and strong coordination effect, the probe is very sensitive to Ca 2+ The measurement is basically not interfered by other coexisting ions.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap