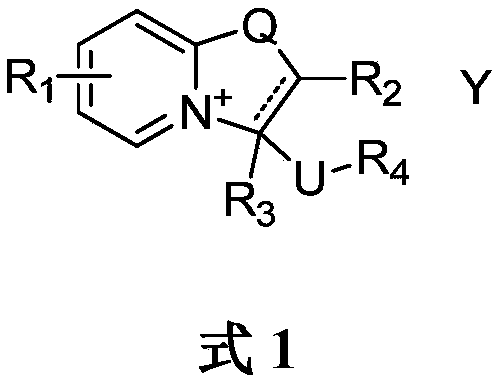
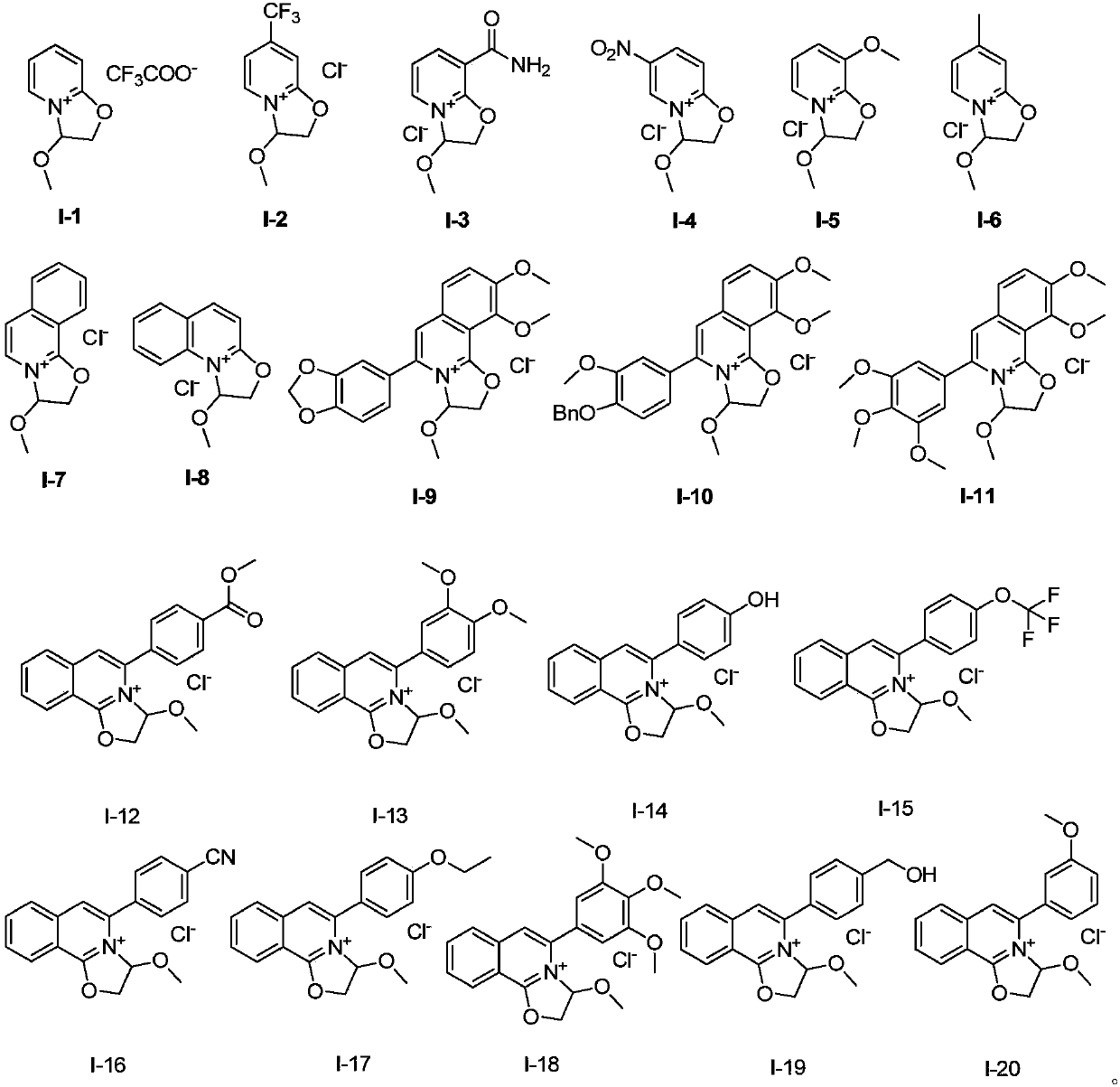
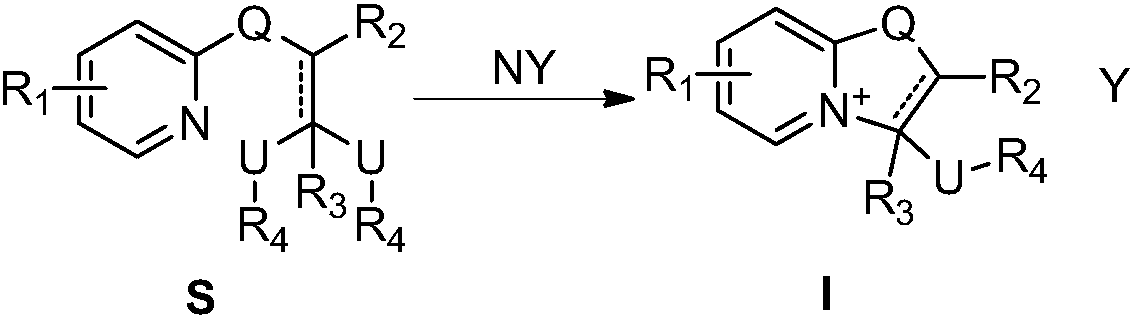
Oxazolopyridine quaternary ammonium salt compound and preparation method and application thereof
A technology of oxazolopyridine and quaternary ammonium salts, which is applied in the transformation application field of oxazolopyridine quaternary ammonium salt compounds and synthetic intermediates, can solve the problem of low yield, achieve convenient post-processing, and good promotion and application value , The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0048] Example 1 Preparation of Compound I-1
[0049]
[0050] Dissolve compound S-1 in dry toluene, add excess trifluoroacetic acid to it, heat to 50℃, keep dry, react overnight, spin-dry the excess acid and solvent, and wash with ether to obtain pure compound I-1 , The yield was 86%. 1 HNMR (400MHz, chloroform-d) δ 7.62 (dd, J = 7.0, 2.0 Hz, 1H), 7.52 (ddt, J = 8.7, 6.6, 2.7 Hz, 1H), 6.75 (d, J = 9.1 Hz, 1H ), 6.47(q,J=7.1Hz,1H), 6.08–5.97(m,1H), 3.78(q,J=11.5,9.8Hz,2H), 3.37(s,3H). 13 C NMR(125MHz, CDCl 3 )δ159.91,149.11,137.12,119.27,110.64,93.01,75.74,58.15.MS(EI):265.
Example Embodiment
[0051] Example 2 Preparation of Compound I-2
[0052]
[0053] The compound S-2 was dissolved in dry anhydrous ether, and an excessive amount of concentrated hydrochloric acid was added to it. A large amount of yellow solid precipitated immediately. After stirring for 3 hours, it was filtered and washed with anhydrous ether to obtain the pure compound I-2. Yield Is 92%. 1 H NMR(400MHz, chloroform-d)δ7.66(d,J=7.3Hz,1H), 6.88(dd,J=1.9,1.0Hz,1H), 6.43(dd,J=7.3,2.0Hz,1H) ,6.07(dd,J=4.8,3.6Hz,1H), 3.87–3.70(m,2H), 3.45(s,3H) 13 C NMR(125MHz, CDCl 3 )δ160.93,141.24,140.97,133.41,122.63,120.45,117.99,100.85,84.91,57.37,43.98.MS(EI)255.
Example Embodiment
[0054] Example 3 Preparation of Compound I-3
[0055]
[0056] The preparation method was the same as that in Example 2, and compound S-2 was replaced with compound S-3 to obtain compound I-3, and the yield was 99%. 1 HNMR(400MHz, chloroform-d)δ9.28(s,1H), 8.60(dd,J=7.2,2.2Hz,1H), 7.77(dd,J=6.8,1.9Hz,1H), 6.56(t,J =6.9Hz,1H), 6.22–6.09(m,1H), 5.97(s,1H), 3.93–3.69(m,2H), 3.46(s,3H). 13 C NMR(125MHz, CDCl 3 )δ164.76,161.45,144.37,135.61,120.97,106.16,85.37,57.49,44.09.MS(ESI)195.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap