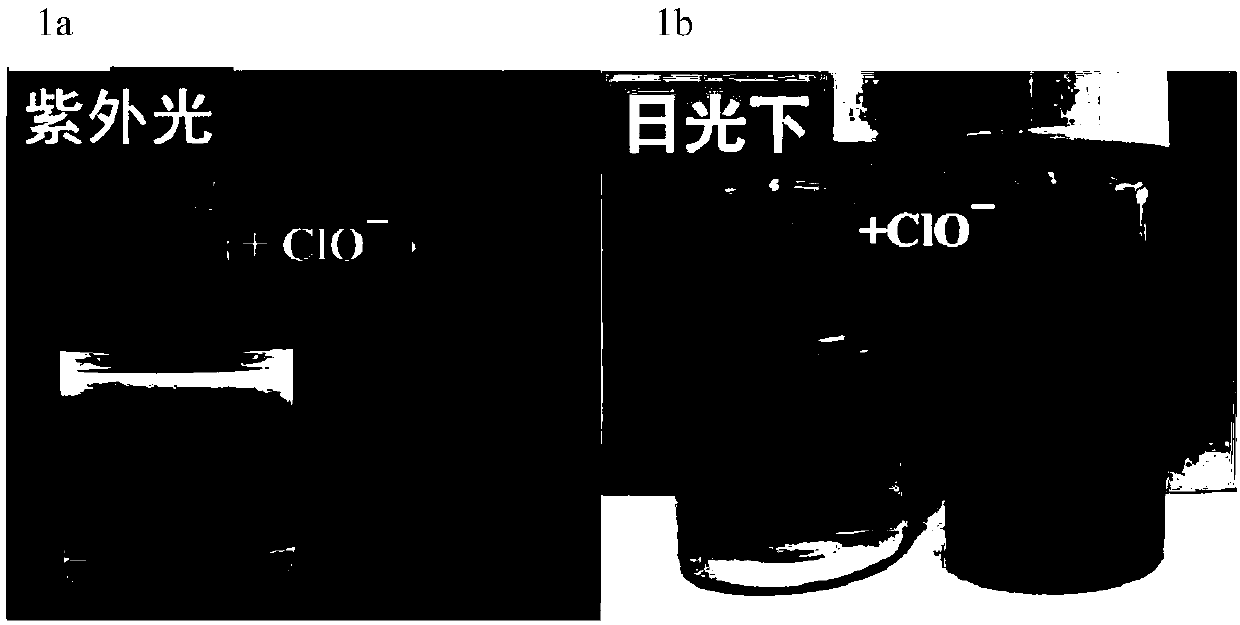
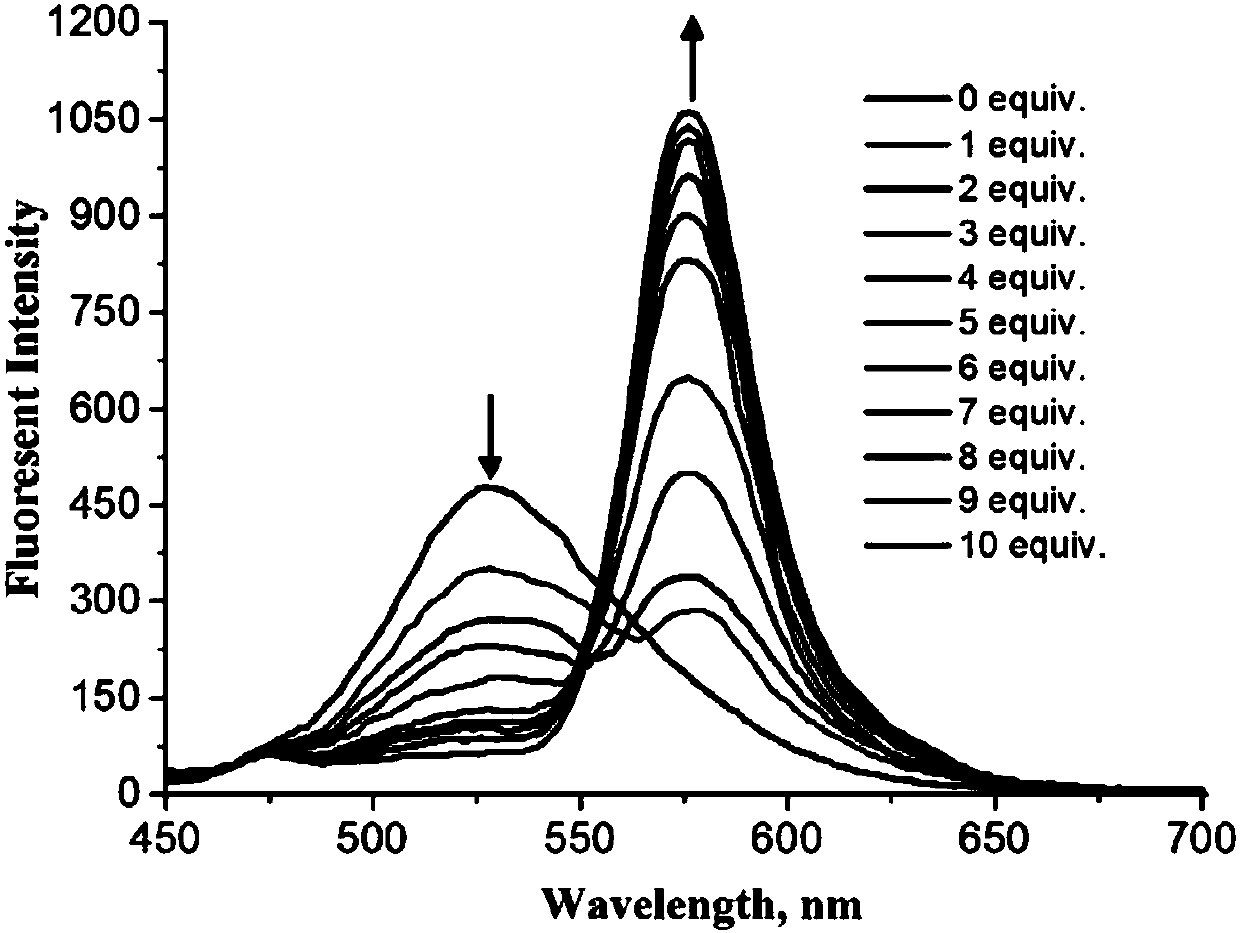
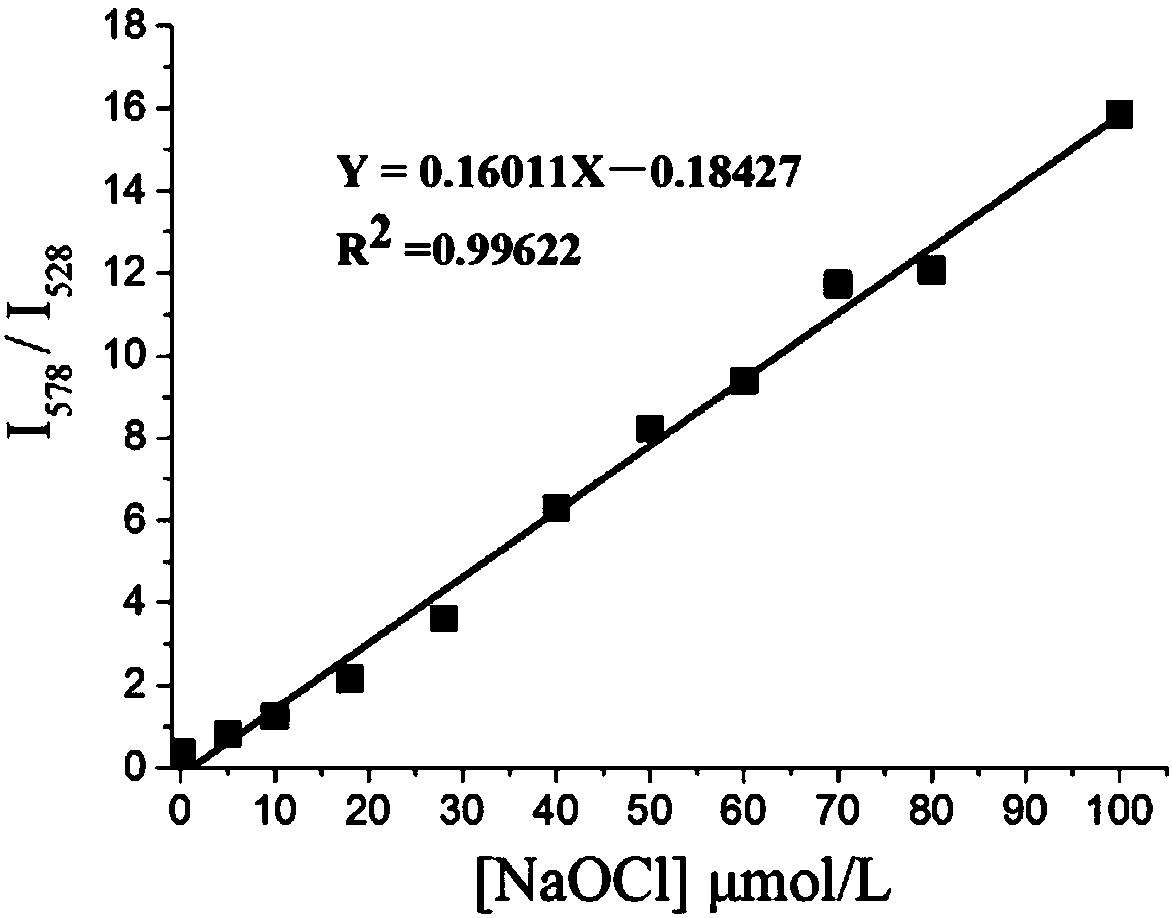
Fluorescence probe molecule as well as preparation method and application thereof to hypochlorite ion detection
A fluorescent probe and synthesis method technology, applied in the field of water sample detection, to achieve the effect of good selectivity, high sensitivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] A method for synthesizing fluorescent probe molecules, comprising the following steps:
[0035] Reaction of 4-bromo-1,8-naphthalic anhydride with methylamine yields compound 2.
[0036] Substitution reaction of compound 2 with hydrazine hydrate yields compound 3.
[0037] After reacting rhodamine B with phosphorus oxychloride, continue to react with compound 3 to generate the fluorescent probe molecule shown in structural formula A.
[0038] The synthetic route is as follows:
[0039]
[0040] Further, the steps of reacting 4-bromo-1,8-naphthalene dicarboxylic anhydride and methylamine to generate compound 2 are:
[0041] Dissolving 4-bromo-1,8-naphthalene dicarboxylic anhydride in methylamine, reacting at 50-100°C for 0.5-2 hours to obtain compound 2. The preferred reaction condition is to react at 10-40° C. for 1.5-3 hours.
[0042] Further, the step of generating compound 3 by substitution reaction of compound 2 with hydrazine hydrate is:
[0043] Compound 2 was
Embodiment 1
[0053] Synthesis of compound 2
[0054]Add 4-bromo-1,8-naphthalic anhydride (5 g, 18 mmol) into a 100 mL round bottom flask, add 54 mL of 33% methylamine solution to dissolve, and stir at 85° C. for 1 h. The reaction was stopped, and the solid crude product was obtained by filtration. After the solid was fully dried, it was recrystallized with chlorobenzene to obtain 4.43 g of off-white compound 2 with a yield of 83%.
[0055] m.p.172-174°C; 1 H NMR (400MHz, CDCl 3 )δ3.56(s,3H),7.86(dd,J=8.4Hz&J=0.8Hz,1H),8.05(d,J=7.6Hz,1H),8.43(d,J=8.0Hz,1H),8.58 (dd, J=8.4Hz&J=1.2Hz,1H),8.67(dd,J=8.4Hz&J=1.2Hz,1H).
[0056] Synthesis of compound 3
[0057] In a 100 mL round bottom flask, compound 2 (2.4 g, 8 mm01) and hydrazine hydrate (80%, 25 mL) were sequentially added, and the reaction was stirred at 130° C. for 1 h. Stop the reaction and cool to room temperature, add a large amount of water to the solution, a yellow solid precipitates, filter and wash with water to obtain 1.66g of
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap