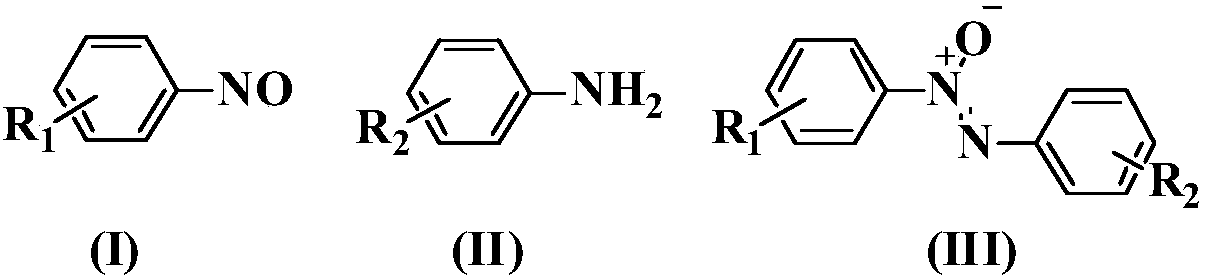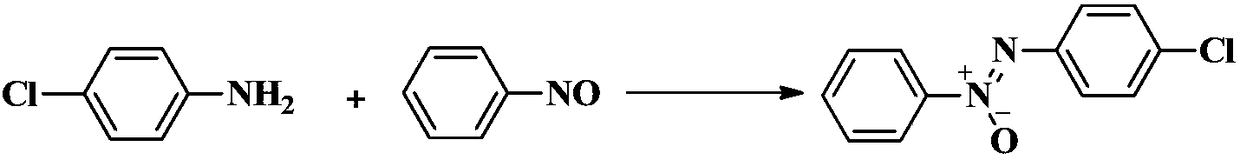Synthesis method of asymmetric azoxybenzene compound
A technology of azobenzene oxide and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, restrictions on extensive research and application, and complex preparation of raw materials, and achieve good product yield, good research value and application potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
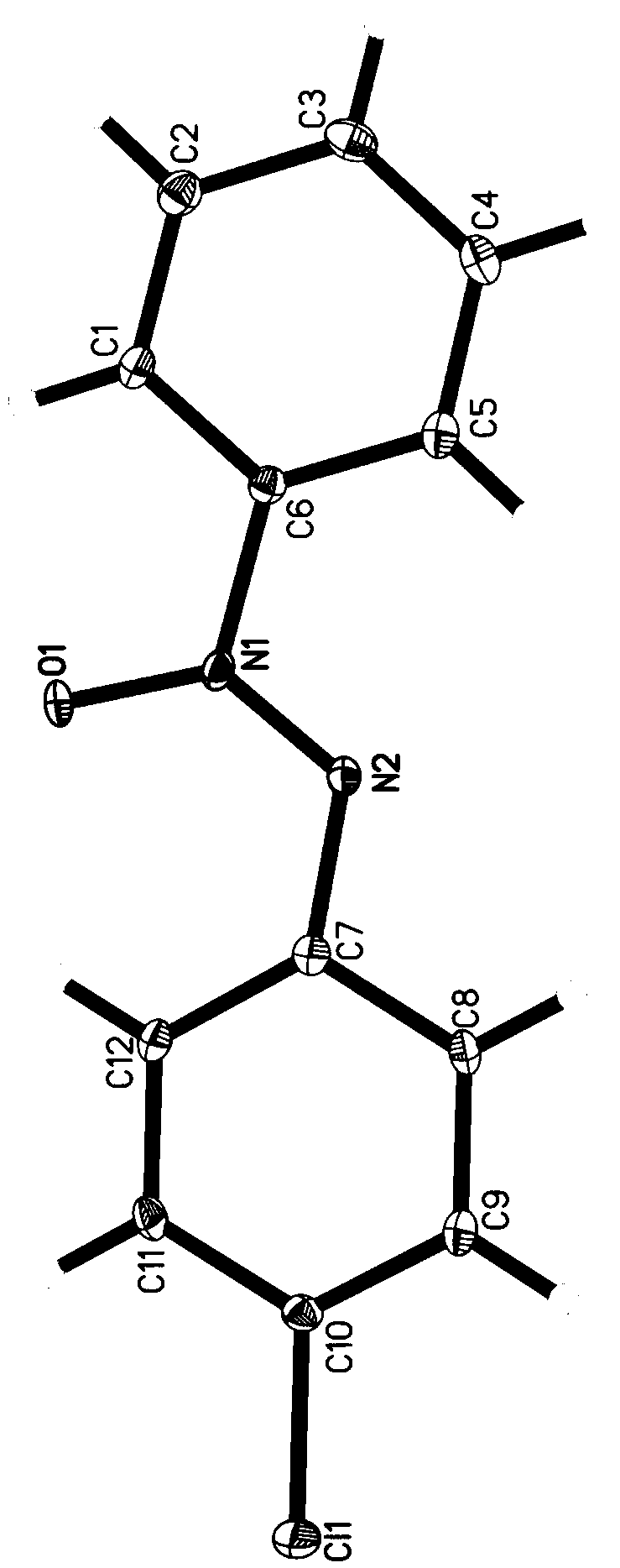
[0029] Example 1 1-phenyl-2-(4-chlorophenyl)azo oxide
[0030]
[0031] 4-Chloroaniline (127.5mg, 1.0mmol), nitrosobenzene (117.8mg, 1.1mmol), Ag 2 O (254.5 mg, 1.1 mmol), DMSO (2 mL). Then, seal the tube. Put the reaction mixture in an oil bath at 65°C and stir for 24 hours, then take it out and cool it to room temperature, transfer the reaction stock solution, add an appropriate amount of ethyl acetate to wash, mix the stock solution with the washing solution, concentrate under reduced pressure and adsorb on silica gel powder, Directly carry out silica gel column chromatography separation, using the mixture of petroleum ether and ethyl acetate with a volume ratio of 100:1 as the eluent, and obtain 1-phenyl-2-(4-chlorophenyl) oxidation after column chromatography separation Azo, yield 85%.
[0032] The structural data confirmed by NMR are: 1 H NMR (500MHz, CDCl 3 , TMS): δ8.29(d, J=8.0Hz, 2H), 8.16(d, J=9.0Hz, 2H), 7.56(t, J=8.0Hz, 1H), 7.51(t, J=8.0Hz ,2H),7.44(d,J=9.0
Embodiment 2
[0033] Example 2 1-phenyl-2-(4-methyl carboxylate phenyl)azo oxide
[0034]
[0035] Add 4-methylaniline formate (151.2mg, 1.0mmol), nitrosobenzene (117.8mg, 1.1mmol), Ag 2 O (254.5 mg, 1.1 mmol), DMSO (2 ml). Then, seal the tube. Put the reaction mixture into an oil bath at 80°C and stir for 24 hours, then take it out and cool to room temperature, transfer the reaction stock solution, add an appropriate amount of ethyl acetate to wash, mix the stock solution with the washing solution, concentrate under reduced pressure and adsorb on silica gel powder, Directly carry out silica gel column chromatography separation, use the mixture of petroleum ether and ethyl acetate with a volume ratio of 50:1 as eluent, obtain 1-phenyl-2-(4-methyl phenyl carboxylate after separation by column chromatography ) azo oxide, yield 71%.
[0036] The structural data confirmed by NMR are: 1 H NMR (500MHz, CDCl3 , TMS): δ8.31(d, J=8.0Hz, 2H), 8.14(s, 4H), 7.58(t, J=7.5Hz, 1H), 7.52(t, J=7.5Hz, 2H
Embodiment 3
[0037] Example 3 1-phenyl-2-(4-iodophenyl)azo oxide
[0038] Add 4-iodoaniline (221.3mg, 1.0mmol), nitrosobenzene (117.8mg, 1.1mmol), Ag 2 O (254.5 mg, 1.1 mmol), DMSO (2 ml). Then, seal the tube. Put the reaction mixture in an oil bath at 65°C and stir for 24 hours, then take it out and cool it to room temperature, transfer the reaction stock solution, add an appropriate amount of ethyl acetate to wash, mix the stock solution and washing solution, concentrate under reduced pressure and adsorb on silica gel powder, Directly carry out silica gel column chromatography separation, using the mixture of petroleum ether and ethyl acetate with a volume ratio of 100:1 as the eluent, and obtain 1-phenyl-2-(4-iodophenyl) oxidation after column chromatography separation Azo, yield 90%.
[0039] The structural data confirmed by NMR are: 1 H NMR (500MHz, CDCl 3 , TMS): δ8.29(d, J=8.0Hz, 2H), 7.92(d, J=8.5Hz, 2H), 7.81(d, J=8.5Hz, 2H), 7.57(t, J=8.0Hz ,1H),7.51(t,J=8.0Hz,2H). 13 C NMR
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap