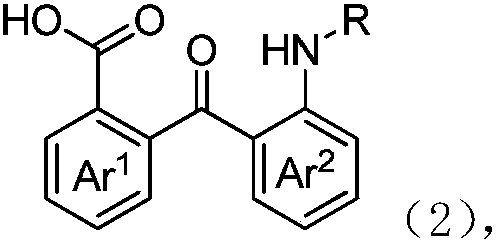
2-(2-aminobenzoyl) benzoic acid derivative and preparing method thereof
An aminobenzoyl and benzoic acid technology, applied in the field of 2-benzoic acid derivatives and their preparation, can solve the problems of poor regioselectivity, difficult synthesis of regioselectivity, etc. wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of N-butyl-2-fluoro-N-(2-fluorobenzyl)benzamide
[0043]
[0044] Add 10 mmol of n-butylamine, 10 mmol of 2-fluorobenzaldehyde, 2 g of anhydrous magnesium sulfate, and 30 mL of dichloromethane into a 100 mL round bottom flask, and stir at room temperature for 2 h to obtain an imine. Filter, concentrate, add 30 mL of methanol, add 10 mmol of sodium borohydride in an ice-water bath, stir at room temperature for 1 h, add 30 mL of water to the reaction solution, extract the mixture with dichloromethane, collect the organic phase, add anhydrous sodium sulfate to dry, and then filter , Concentrate to obtain the corresponding amine. The obtained amine was mixed with 12mmol of 2-fluorobenzoic acid, 12mmol of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI), 12mmol of 1-hydroxybenzotriazole (HOBt) 12mmol and 20mmol of diisopropylethylamine were dissolved in 20mL N,N-dimethylformamide, and stirred at room temperature for 24h. Add an appropriate amo
Embodiment 1-13
[0045] Following embodiment 1-13 is the preparation method of 2-(2-aminobenzoyl) benzoic acid derivative
[0046] Example 1
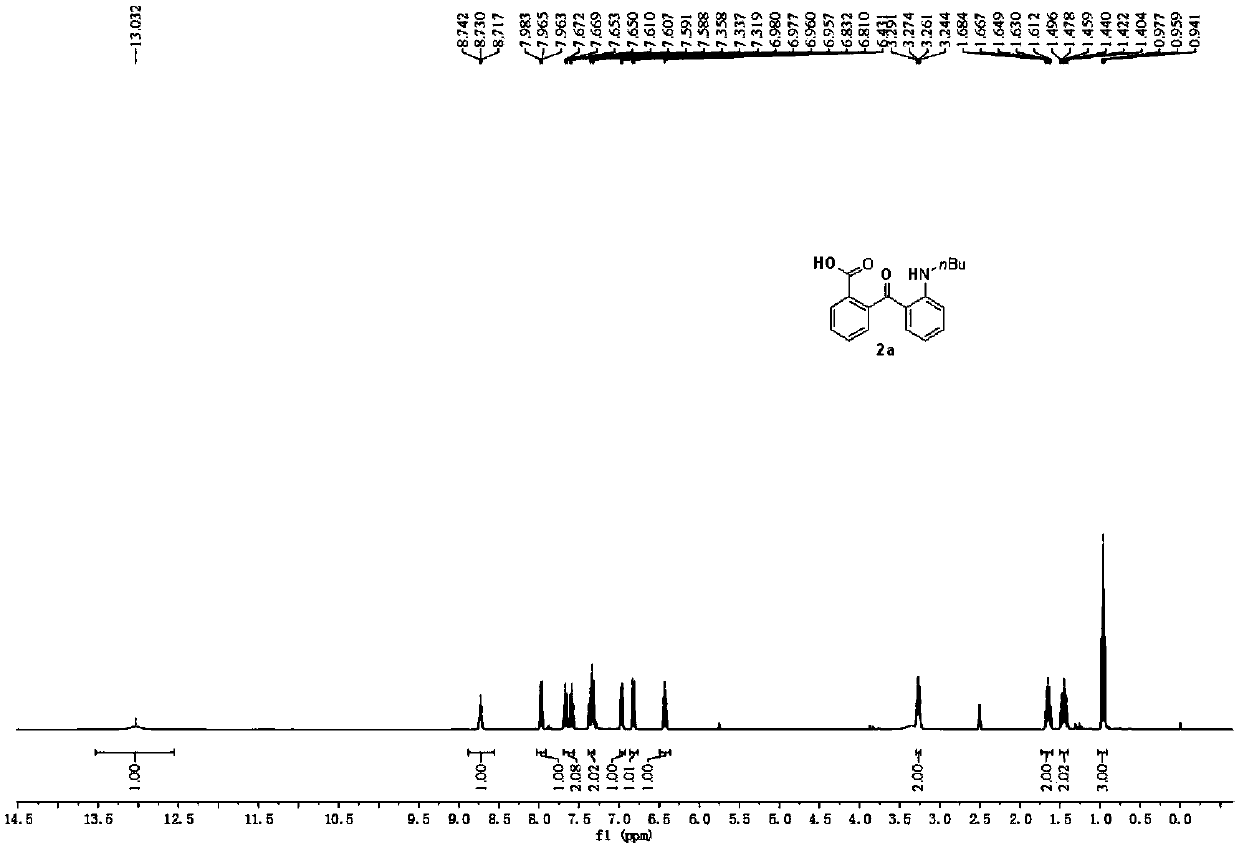
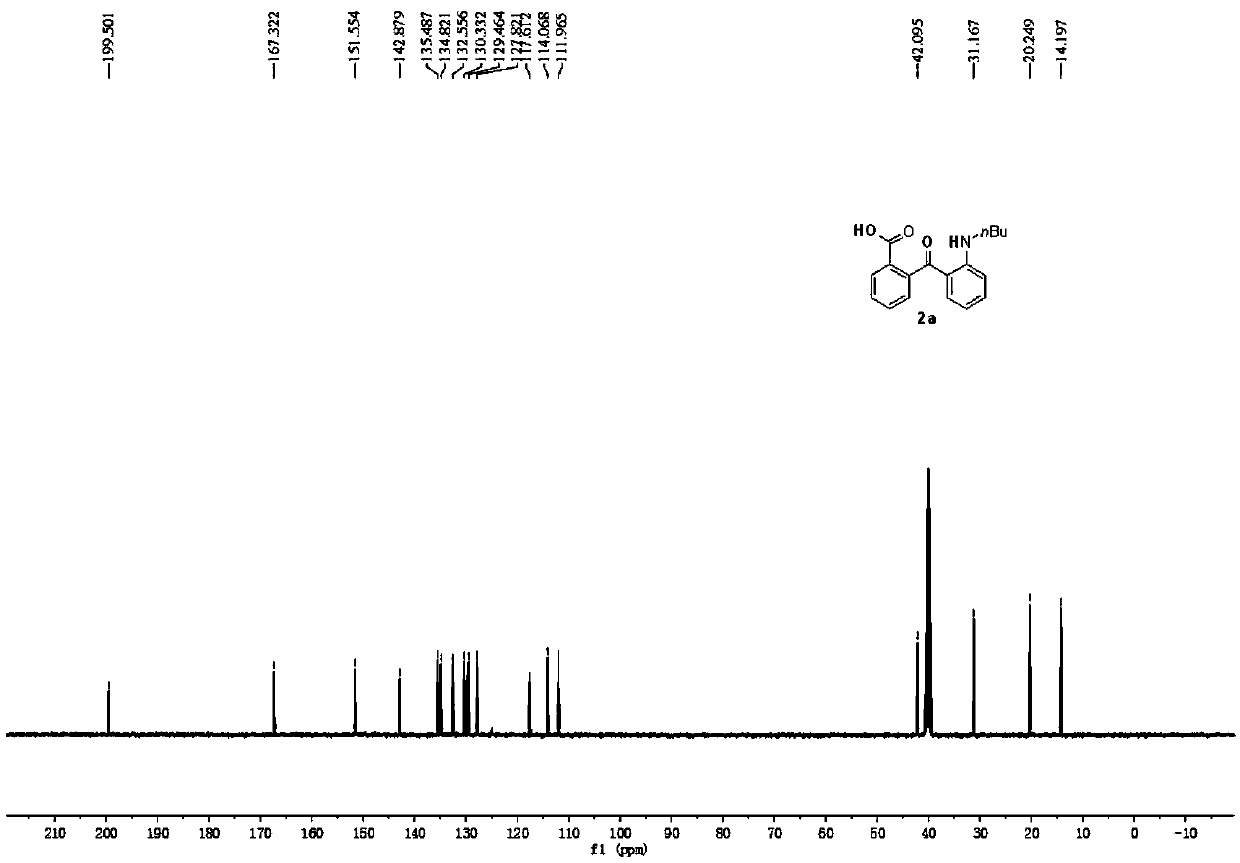
[0047] Preparation of 2-(2-(N-butylamino)benzoyl)benzoic acid
[0048]
[0049] Put 0.5mmol of N-butyl-2-fluoro-N-(2-fluorobenzyl)benzamide (1a), 2mmol of potassium hydroxide, and 10mL of dimethylsulfoxide in an oil bath at 100°C, and react in air 5h. Add appropriate amount of water or sodium chloride solution to stop the reaction, and cool to room temperature. The pH of the reaction solution was adjusted to acidity, diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by column chromatography to obtain 120.3 mg of the target product with a yield of 81%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, DMSO) δ13.03(s, 1H), 8.73(t, J=5.1Hz, 1H), 8.03–7.91(m, 1H), 7.63(dtd, J=32.4, 7.5, 1.1Hz, 2H), 7.38–7.31(m, 2H), 6.97(dd,
Embodiment 2
[0051] Preparation of 2-(2-(N-butylamino)benzoyl)-4-methylbenzoic acid
[0052]
[0053] Place N-butyl-2-bromo-N-(2-fluorobenzyl)-4-methylbenzamide (1b) 0.5mmol, sodium tert-butoxide 1.5mmol, N-methylpyrrolidone 2mL in 80 ℃ oil bath, the reaction 12h. Add appropriate amount of water or sodium chloride solution to stop the reaction, and cool to room temperature. The pH of the reaction solution was adjusted to acidity, diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by column chromatography to obtain 96.4 mg of the target product with a yield of 62%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, DMSO) δ12.84(s, 1H), 8.71(t, J=4.8Hz, 1H), 7.86(d, J=8.0Hz, 1H), 7.36(dd, J=17.5, 8.2Hz ,2H),7.13(s,1H),6.96(d,J=7.9Hz,1H),6.81(d,J=8.5Hz,1H),6.42(t,J=7.5Hz,1H),3.26(dd ,J=12.4,6.7Hz,2H),2.38(s,3H),1.73–1.55(m,2H),1.45(dq,J=14.5,7.3Hz,2H),0.96(t,J=7.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap