Preparation and application of mycoplasma pneumoniae recombinant antigen
A technology of mycoplasma pneumoniae and recombinant antigen, which is applied in the field of bioengineering, can solve the problems of increasing the infection probability of experimental operators, cumbersome production process of natural antigen, unfavorable treatment of patients, etc. The method is convenient and fast, suitable for popularization and application, and has good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
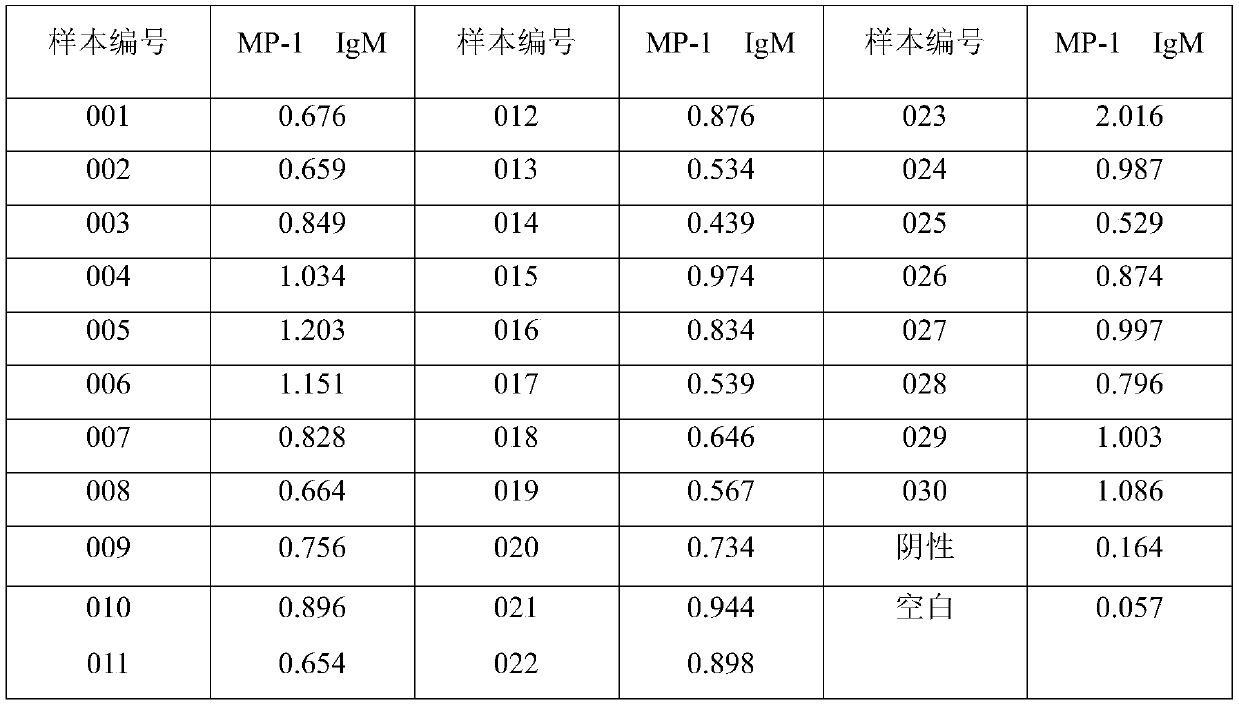
Image
Examples
Embodiment 2
[0037] Application of Recombinant Mycoplasma Pneumoniae Antigen
[0038] (1) Coating protein is high-purity gene recombinant MP protein
[0039] The coating protein is the high-purity gene recombinant MP-1 protein and MP-2 prepared in Example 1.
[0040] (2) Preparation of antigen-coated microtiter plates
[0041] Dilute MP protein 1:10000 with coating buffer (pH value 9.6, 0.1mol / L carbonate buffer), add to the wells of the microplate, 100 μL per well, react at 37°C for 2 hours, shake After removing the coating solution, pat dry, add 200 μL of blocking solution (containing 2% bovine serum albumin phosphate buffer at a final concentration) to each well, react at 37°C for 2 hours, shake off the blocking solution, pat dry, dry, and use Store in aluminum foil bag vacuum packaging.
[0042] 2. Preparation of working concentration enzyme solution
[0043] HRP-labeled goat anti-human IgM needs to be purified by affinity chromatography, with strong species specificity, a purity gr
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap