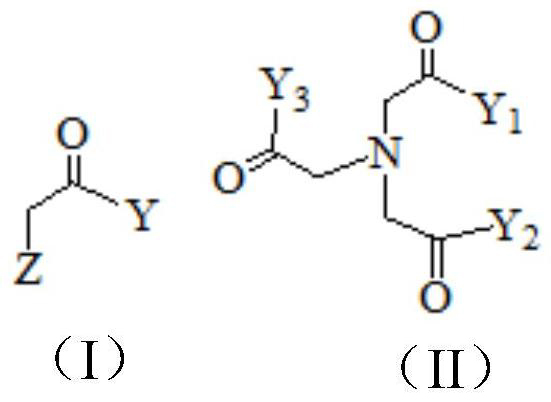
Preparation process of N-(2-acetamido)-2-iminodiacetic acid
A technology of iminodiacetic acid and acetamide, which is applied in the field of preparation technology of N--2-iminodiacetic acid, can solve the problems of ADA being expensive and rare, and achieve shortened reaction production cycle, easy control, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of preparation technology of N-(2-acetamido)-2-iminodiacetic acid (ADA), comprising:
[0041] Step 1: In the reactor, in the presence of the reaction solvent acetonitrile, add ammonia water and methyl chloroacetate at a molar ratio of 1:1, set the reaction temperature to 20°C, and the reaction pressure to normal pressure. After 8 hours, the reaction is complete, and Intermediate products, as follows:
[0042]
[0043] Then spin off the solvent acetonitrile, add ethyl acetate, wash twice with water, dry over anhydrous sodium sulfate, then spin off the ethyl acetate to obtain an intermediate product with higher purity, the yield of the intermediate product is calculated to be 80%.
[0044]Step 2: In the presence of the reaction solvent acetonitrile, add amination reagent ammonia water to the reactor to react with the intermediate product obtained in step 1, wherein the molar ratio of ammonia water to the intermediate product is 1:1, and the reaction temperature is
Embodiment 2
[0054] A kind of preparation technology of N-(2-acetamido)-2-iminodiacetic acid (ADA), comprising:
[0055] Step 1: In the reactor, in the presence of the reaction solvent tetrahydrofuran, add ammonium chloride and chloroacetic acid at a molar ratio of 1:1, set the reaction temperature to 20°C, and the reaction pressure to normal pressure. After 8 hours, the reaction is complete, and Intermediate products, as follows:
[0056]
[0057] Then the solvent tetrahydrofuran was spun off, ethyl acetate was added, washed twice with water, dried over anhydrous sodium sulfate, and then the ethyl acetate was spun off to obtain an intermediate product with higher purity. The yield of the intermediate product was calculated to be 70%.
[0058] Step 2: In the presence of the solvent tetrahydrofuran, add ammonia as an aminating reagent to the reactor to react with the intermediate product obtained in step 1, wherein the molar ratio of ammonia water to the intermediate product is 1:1, and the
Embodiment 3
[0068] A kind of preparation technology of N-(2-acetamido)-2-iminodiacetic acid (ADA), comprising:
[0069] Step 1: In the reactor, in the presence of the reaction solvent methanol, add ammonium nitrate and ethyl chloroacetate at a molar ratio of 1:1, set the reaction temperature to 20°C, and the reaction pressure to normal pressure, and the reaction is completed after 8 hours. An intermediate product is generated, as follows:
[0070]
[0071] Then the solvent methanol was spun off, ethyl acetate was added, washed twice with water, dried over anhydrous sodium sulfate, and then ethyl acetate was spun off to obtain an intermediate product with higher purity. The yield of the intermediate product was calculated to be 85%.
[0072] Step 2: In the presence of solvent methanol, add amination reagent ammonia water to the reactor to react with the intermediate product obtained in step 1, wherein the molar ratio of ammonia water to intermediate product is 1:1, and the reaction tempera
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap