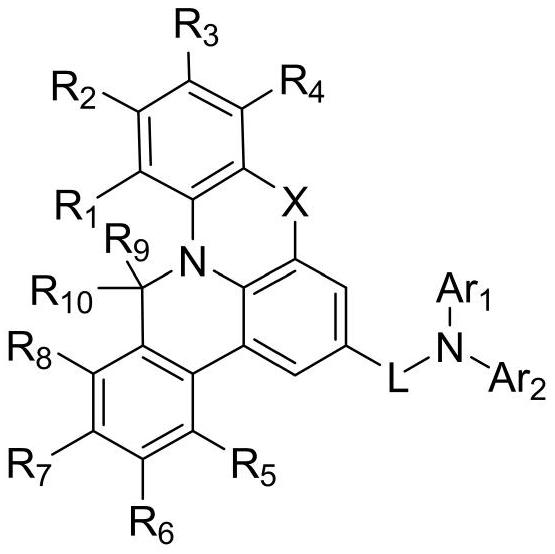
Organic electroluminescent compound and preparation method thereof
A compound and luminescent technology, applied in the direction of silicon organic compounds, organic chemistry, chemical instruments and methods, etc., can solve the problems of low glass transition temperature, poor thermal stability, reduced lifespan, etc., and achieve simple synthesis steps and high practicality Promotion significance and application value, the effect of not demanding conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of Compound 1:
[0047]
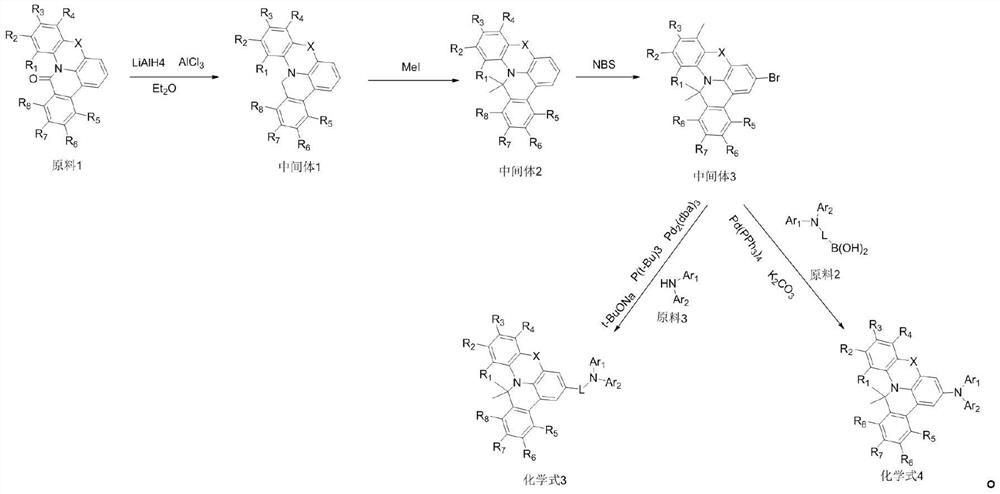
[0048] Specifically: add raw material 1 (37mmol) and 215ml anhydrous ether in the reaction vessel, pass into N 2 , at N 2 Slowly add AlCl under protection 3 (46mmol) stirred for 15min, cooled to 0°C, slowly added LiAlH 4 (56.6mmol) was warmed up to 50°C, stirred for 1h, and the reaction was completed; then 10ml of EA and 20ml of 6mol / L hydrochloric acid solution were added dropwise at ice-water bath temperature, and the reaction solution was extracted with DCM; then the extracted organic layer, and the solvent was removed using a rotary evaporator; intermediate 1 (6.2 g, yield 65.7%, MW: 255.32) was obtained by precipitation with DCM and ethanol;
[0049] Intermediate 1 (24.3mmol) and t-BuOk (170.1mmol) and 200mlTHF were added to the reaction vessel, stirred for 30min, added MeI (212.5mmol) and raised to 40°C, stirred for 12h, and the reaction was complete; followed by dichloromethane and The mixture was extracted with water;
Embodiment 2
[0053] Preparation of compound 12:
[0054]
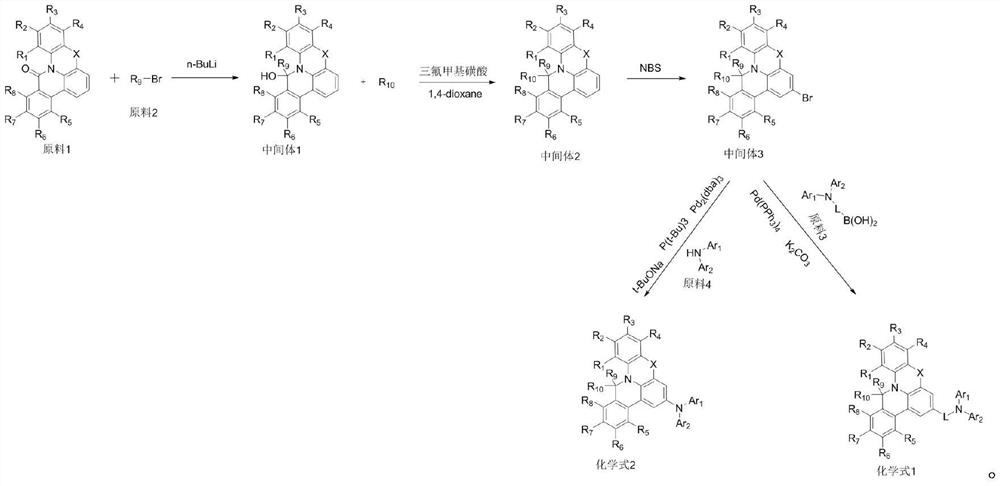
[0055] Add bromobenzene (44.4mmol) and 100mlTHF in the reaction vessel, ventilate 3 times and cool to-78 ℃, under N 2 Add n-BuLi (44.4mmol) under atmosphere and stir for 2h, add raw material 1 (10g, 37mmol) and raise the temperature to 25°C, stir for 10h, and the reaction is complete; then distilled water is added to the reaction solution to quench the reaction, and the reaction is extracted with DCM Solution, then use magnesium sulfate to dry the extracted organic layer, and use a rotary evaporator to remove the solvent, use DCM and PE (1:5) to separate out the solid to obtain intermediate 1 (8g, yield 62%, MW:347.42);
[0056] Intermediate 1 (23mmol) and benzene (23mmol) were dissolved in 150mL 1,4-dioxane and ventilated 3 times under N 2 Add trifluoromethanesulfonic acid (115mmol) under atmosphere and raise the temperature to 120°C, stir for 12h, and the reaction is complete; then extract the mixture with dichloromethane and wa
Embodiment 3
[0060] Preparation of Compound 21:
[0061]
[0062] Add raw material 2 (44.4mmol) and 100mlTHF to the reaction vessel, ventilate 3 times and cool down to -78°C, under N 2 Add n-BuLi (44.4mmol) under atmosphere and stir for 2h, add raw material 1 (10g, 37mmol) and raise the temperature to 25°C, stir for 10h, and the reaction is complete; then distilled water is added to the reaction solution to quench the reaction, and the reaction is extracted with DCM solution. The extracted organic layer was then dried using magnesium sulfate, and the solvent was removed using a rotary evaporator, and the solid was precipitated with DCM and PE (1:5) to obtain intermediate 1 (10 g, yield 77.82%, MW: 347.40);
[0063] Intermediate 1 (23mmol) and raw material 3 (23mmol) were dissolved in 150mL 1,4-dioxane and ventilated for 3 times, under N 2 Add trifluoromethanesulfonic acid (115mmol) under atmosphere and raise the temperature to 120°C, stir for 12h, and the reaction is complete; then extra
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap