Application of porphyrin in tin in preparation of medicines for treating thalassemia
A technology for treating thalassemia and drugs, applied in the field of medicine, to achieve the effects of alleviating the imbalance of α/β globin chains, improving life expectancy, reducing iron content and splenomegaly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
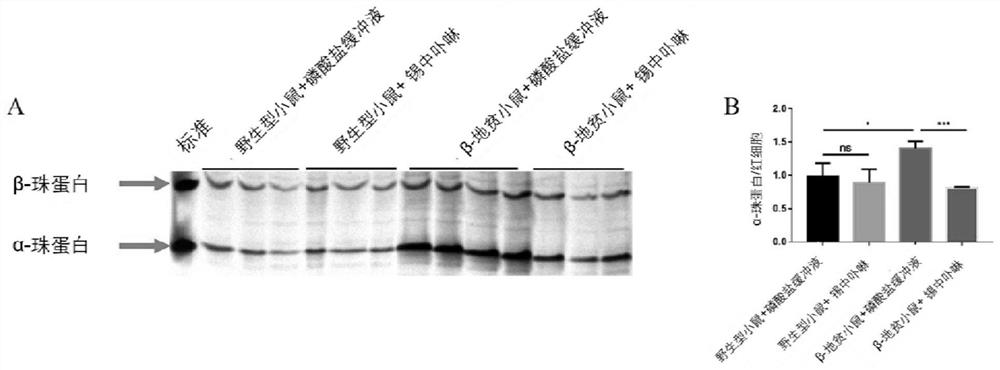
[0020] Example 1: In this example, TAU gel electrophoresis technology was used to detect the effect of SnMP on the deposition of α-globin in peripheral blood erythrocytes of mice
[0021] Experimental steps:
[0022] 1. Divide wild-type (purchased from Hunan Slack Jingda Company) and β-thalassemia mice (purchased from Jackson Laboratory, see references for model construction) of comparable age and sex into 2 groups: one group was intraperitoneally injected with 200 μL Phosphate buffer saline (PBS) with pH 7.2-7.4 was used as the control group, and 200 μL of SnMP was injected intraperitoneally into one group as the experimental group. The dose of SnMP treatment was 25 μmol / kg, intraperitoneal injection 3 times / week, and continued for 4 weeks, and then maintained once a week.
[0023] 2. Preparation of red blood cell membrane samples;
[0024] 1) Take 4 μL of mouse blood with an anticoagulant centrifuge tube;
[0025] 2) Centrifuge the blood cells and wash once with pre-cool
Embodiment 2
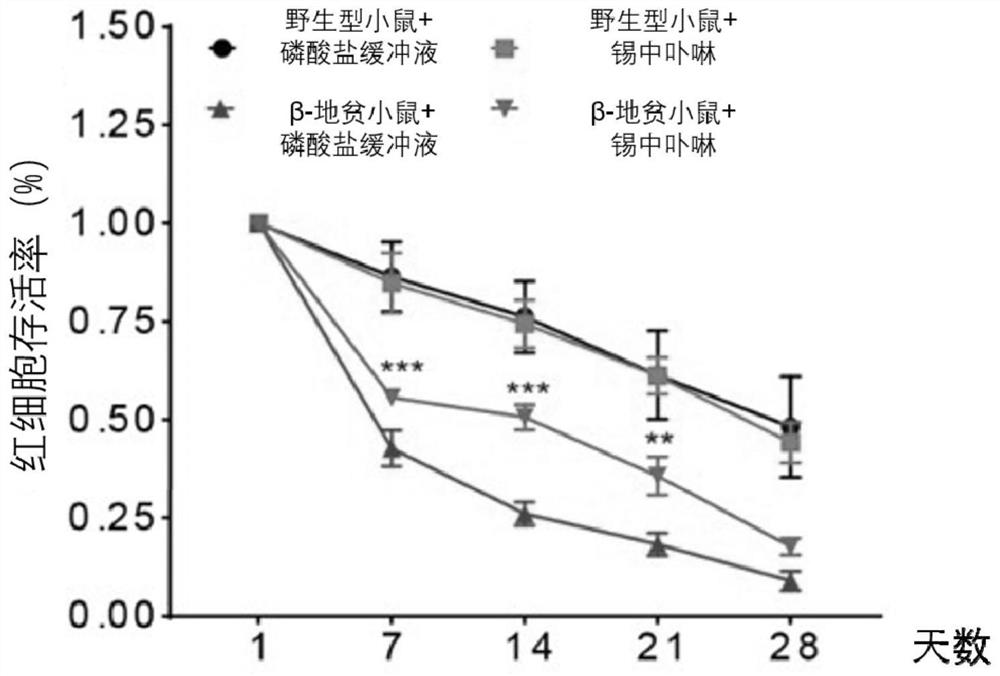
[0031] Example 2: This example uses flow cytometry to detect the effect of SnMP on the half-life of peripheral blood erythrocytes in mice after biotin labeling
[0032] Experimental steps:
[0033] 1. Grouping and treatment of mice are the same as in Example 1;
[0034] 2. Collect 200 μL of peripheral blood from mice in the PBS / SnMP treatment group in an anticoagulant centrifuge tube;
[0035] 3. Add 300 μM biotin labeling solution, mix gently, and incubate at 37°C for 60 minutes in the dark;
[0036] 4. Wash the cells once after labeling, and inject the labeled red blood cells into the wild-type recipient mice through the orbital vein;
[0037] 5. Starting from 24 hours of cell reinfusion into recipient mice, take 2-3 μL of peripheral blood every 7 days, add phycoerythrin (PE)-coupled streptavidin, incubate in the dark for half an hour and then flow up The ratio of PE-positive cells was detected by the instrument, and the results were analyzed and statistically analyzed.
Embodiment 3
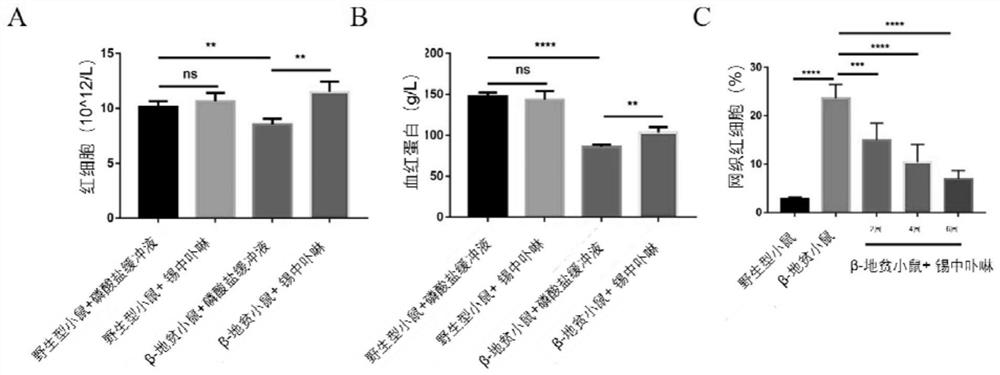
[0039] Example 3: In this example, blood routine is used to detect the influence of SnMP on peripheral blood anemia indicators in mice
[0040] 1. Grouping and treatment of mice are the same as in Example 1;
[0041] 2. Anticoagulant centrifuge tubes were used to collect the peripheral blood of mice treated with PBS / SnMP for 2-6 weeks;
[0042] 3. Dilute the peripheral blood with a diluent for 6 times volume blood cell analysis, and read the data after loading the sample on a blood analyzer (Sysmex Corporation, Japan).
[0043] Experimental results such as image 3 Shown: SnMP treatment 4-6 weeks, thalassemia mice peripheral blood anemia index red blood cell count ( image 3 Middle A) and hemoglobin level ( image 3 Middle B) There is obvious recovery; In addition, the number of reticulocytes in the peripheral blood of thalassemia mice has significantly decreased since SnMP treatment for 2 weeks ( image 3 Middle C), and decreased with the prolongation of treatment time. T
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap