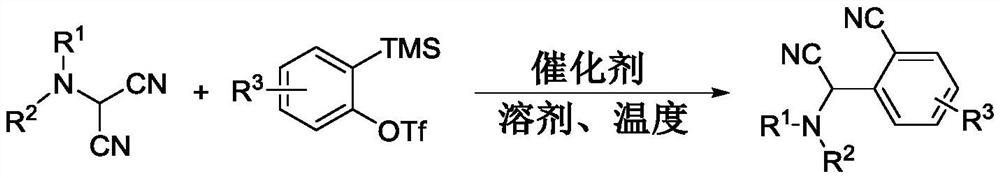
Synthesis method of N-(cyano (2-cyano substituted phenyl) methyl) substituted tertiary amine
A cyano-substituted, tertiary amine technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as cumbersome operation steps and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
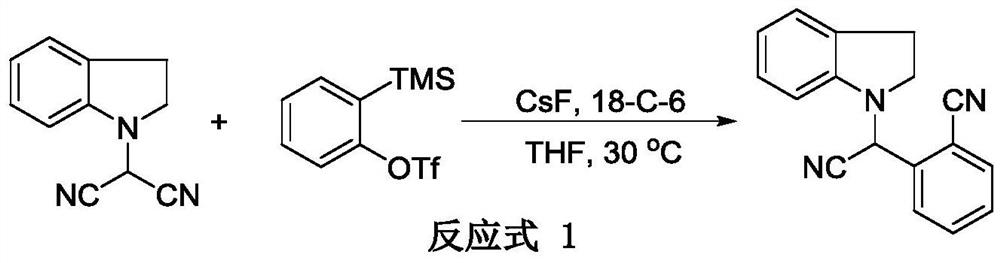
[0016] 1. Using indolinylamino-malononitrile and 2-(trimethylsilyl)phenyl trifluoromethanesulfonate as raw materials (reaction formula 1)
[0017]
[0018] Under the protection of argon, in a 10mL reaction tube, indolinylamino-malononitrile (100mg, 0.55mmol), 2-(trimethylsilyl)phenyltrifluoromethanesulfonate (244mg, 0.82 mmol), cesium fluoride (165mg, 1.09mmol) and 18-crown-6 (289mg, 1.09mmol), were sequentially added to stirred tetrahydrofuran (2mL), reacted at 30°C for 2 hours to complete the reaction, and The solvent was sucked dry on the instrument, and a white solid (106 mg, 75% yield) was obtained by column chromatography.
[0019] The product detection data are as follows:
[0020] 1 H NMR (400MHz, CDCl 3 )δ7.91(d, J=7.6Hz, 1H), 7.79(d, J=7.6Hz, 1H), 7.73(t, J=7.6Hz, 1H), 7.57(t, J=7.6Hz, 1H) ,7.17(t,J=7.2Hz,2H),6.87(t,J=7.2Hz,1H),6.78(d,J=8.0Hz,1H),5.97(s,1H),3.44-3.37(m, 1H),3.17-3.12(m,1H),3.09-3.02(m,1H),3.00-2.91(m,1H); 13 C NMR (100MHz, CDCl 3 )δ148.29,136.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap