Vector and method for expressing L-aspartic acid-alpha-decarboxylase by recombinant Escherichia coli
A technology of recombinant Escherichia coli and aspartic acid, which is applied in the biological field to achieve the effect of increasing protein content and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0104] Example 1:
[0105] 1) Comparative culture of double-plasmid co-expression strain pET32a-pCDFDuet-ADC-BL21(DE3) and single-plasmid strain pET32a-ADC-BL21(DE3) shake flask cell fermentation
[0106] Co-express pET32a-pCDFDuet-ADC-BL21(DE3): Glycerol tube 100μL → 10mL test tube LB liquid medium (add 100mg / L ampicillin and 50mg / L streptomycin), 30°C, 150RPM overnight culture, transfer 5mL → 500mL TB shake flask fermentation medium filling volume 200mL (add 100mg / L ampicillin and 50mg / L streptomycin), 37 ° C, 180RPM for 6 hours → add 10g / L α-lactose induction, induction temperature 30°C→After 17 hours of induction, centrifuge to collect cells→Take 1g of centrifuged wet cells and add 10g of deionized water to mix well→Sonicate and centrifuge, take the crushed supernatant and crushed solution to precipitate, add deionized water to dilute the reset solution ten times, and run proteins separately Electrophoresis SDS-PAGE detection protein expression.
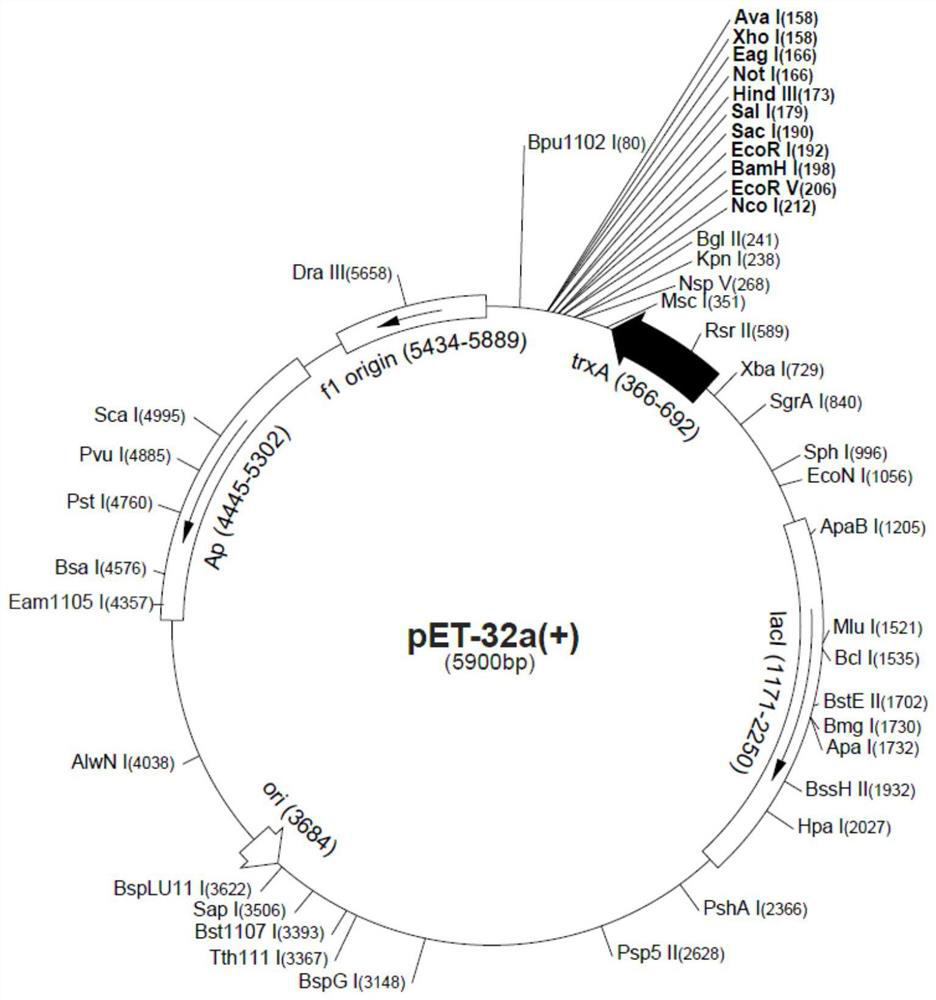
[0107]pET32a-ADC
Example Embodiment
[0112] Example 2:
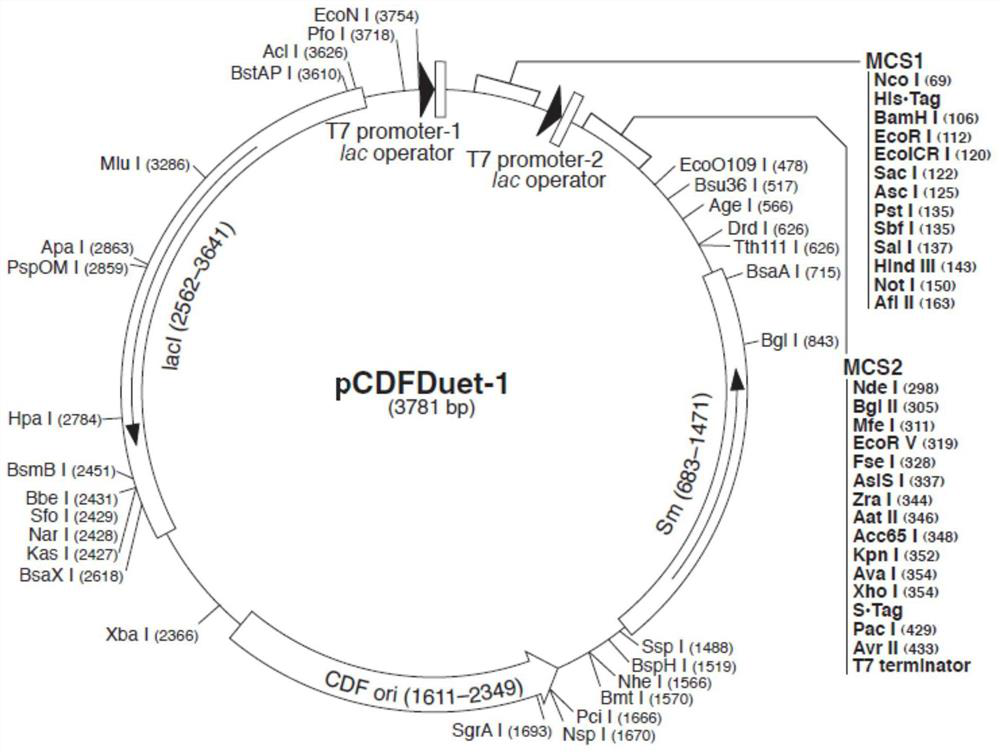
[0113] 1) Comparative culture of double-plasmid co-expression strain pET32a-pCDFDuet-ADC-BL21(DE3) and single-plasmid strain pCDFDuet-ADC-BL21(DE3) shake flask cell fermentation
[0114] pET32a-pCDFDuet-ADC-BL21(DE3): Glycerol tube 100μL→10mL test tube LB liquid medium (add 100mg / L ampicillin and 50mg / L streptomycin), 30℃, 150RPM overnight culture, transfer 5mL→500mL The volume of the TB shake flask fermentation medium is 200mL (add 100mg / L ampicillin and 50mg / L streptomycin), 37°C, 180RPM for 6 hours → add 10g / L α-lactose induction, induction temperature 30°C → After 17 hours of induction, centrifuge to collect cells → Take 1g of centrifuged wet cells and add 10g of deionized water to mix them → Ultrasonic crushing and centrifugation, take the crushed supernatant and the precipitate of the crushed liquid, add deionized water to dilute the reset solution ten times, and run protein electrophoresis SDS-PAGE detection Protein expression.
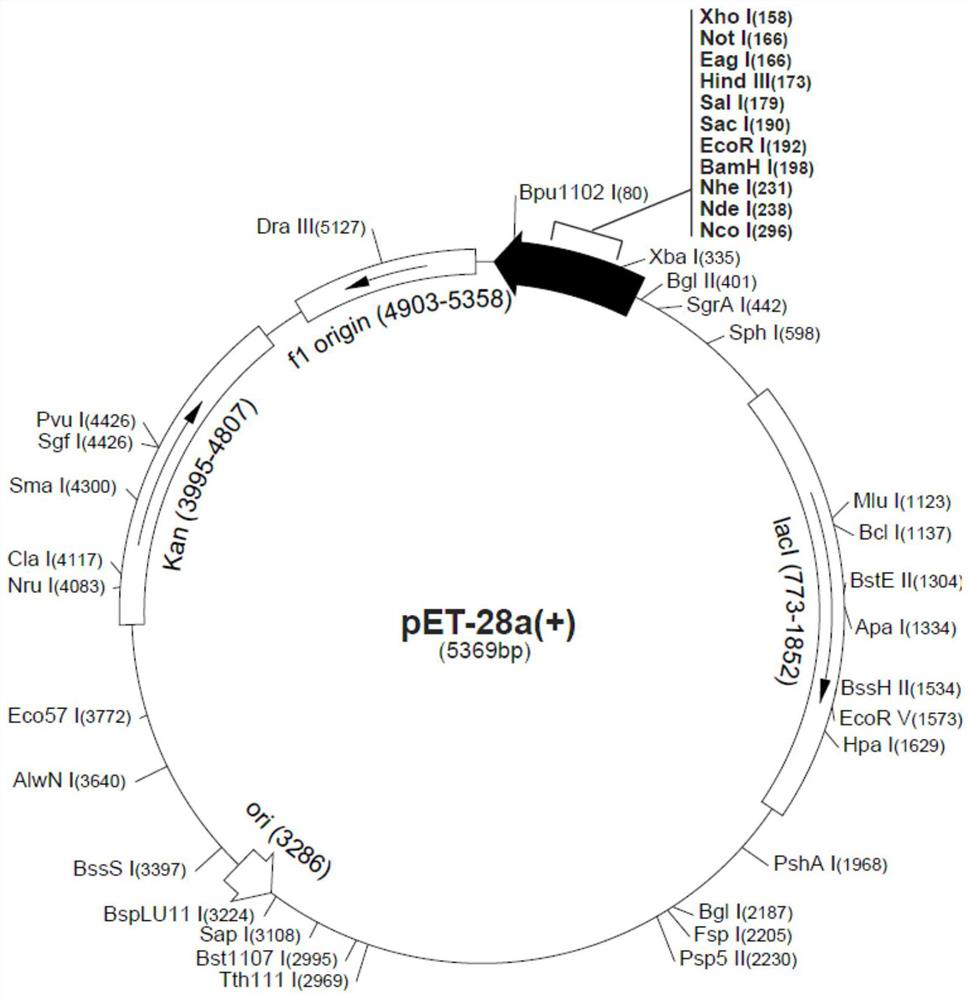
[0115] pCDF
Example Embodiment
[0119] Example 3:
[0120] 1) The three-plasmid co-expression strain pET28a-pET32a-pCDFDuet-ADC-BL21(DE3) and the double-plasmid strain pET32a-pCDFDuet-ADC-BL21(DE3) were compared and cultured in shake flask cells
[0121] Two-plasmid co-expression of pET32a-pCDFDuet-ADC-BL21(DE3): Glycerol tube 100μL → 10mL test tube LB liquid medium (add 100mg / L ampicillin and 50mg / L streptomycin), 30°C, 150RPM overnight culture, transfer Connect 5 mL → 500 mL of TB shake flask fermentation medium with a liquid volume of 200 mL (add 100 mg / L ampicillin and 50 mg / L streptomycin), 37 ° C, 180 RPM for 6 hours → add 10 g / L α-lactose to induce, Induction temperature at 30°C → End of induction for 17 hours and centrifuge to collect cells → Take 1g of centrifuged wet cells and add 10g of deionized water to mix well → Ultrasonic crushing and centrifugation, take the crushed supernatant and crushed liquid to precipitate, add deionized water to dilute the reset solution ten times, respectively
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap