Whole blood simulation liquid for in-vitro test of hollow fiber blood purification device
A technology of blood purification and in vitro testing, applied in the field of whole blood simulated liquid, which can solve the problems of short storage period, low data repeatability, and increased research and development costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The composition of the whole blood simulated liquid (substitute whole blood) is as follows:
[0046] Polystyrene microspheres with a diameter of 6 μm and a simulated liquid with a volume ratio of 28:100, the simulated liquid includes 30wt% bovine serum albumin, 3wt% myoglobin and 1wt% lysozyme, and the balance is water.
[0047] Whole bovine blood was used as the control group.
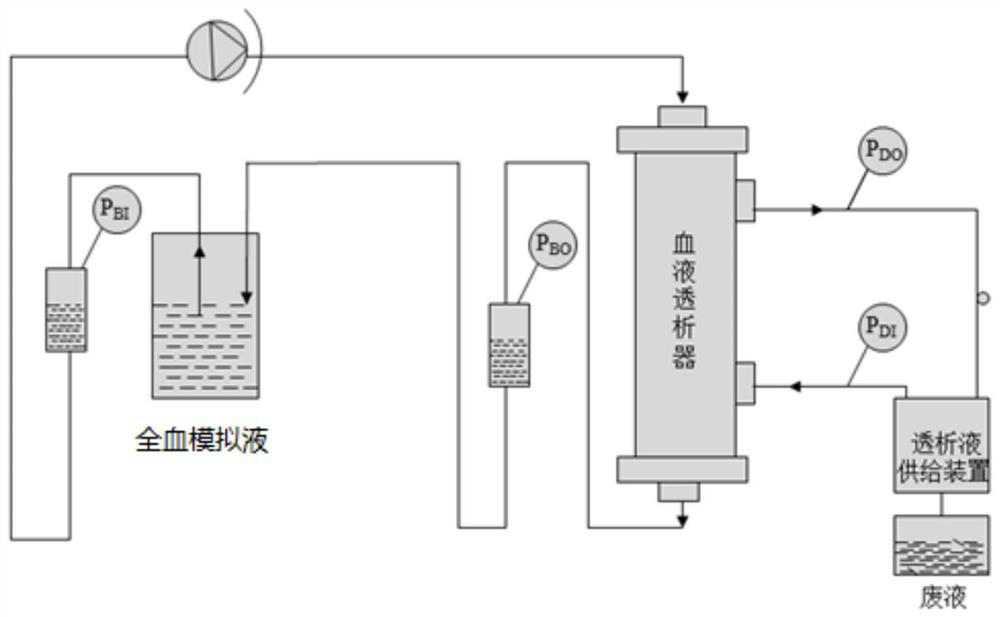
[0048] The present invention adopts figure 1 The shown monitoring device changes with time the transmembrane pressure of whole blood simulated liquid and bovine whole blood, and the test data are shown in Table 1;
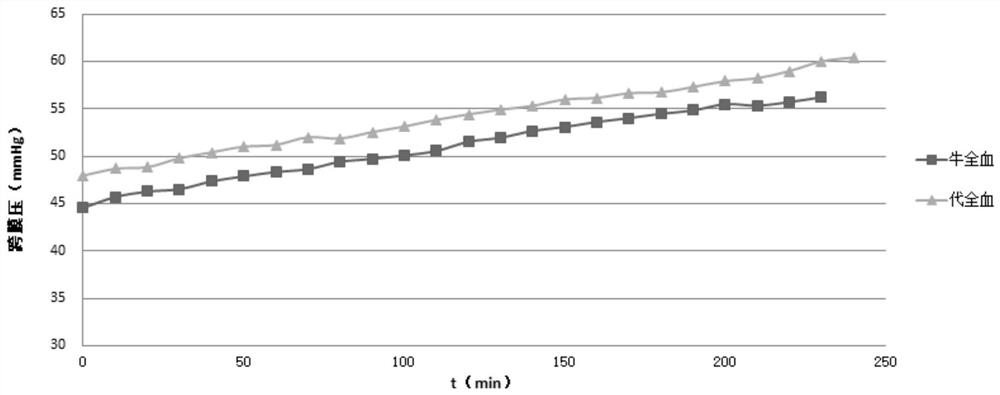
[0049] Table 1 The transmembrane pressure of the whole blood simulated fluid of Example 1 changing over time
[0050] time / min Transmembrane pressure of bovine whole blood (mmHg) The transmembrane pressure (mmHg) of embodiment 1 0 44.6 48.0 10 45.7 48.7 20 46.3 48.8 30 46.5 49.8 40 47.4 50.4 50 47.9 51.0 60 48.3 51.2 70 4
Embodiment 2
[0053] The composition of the whole blood simulated liquid (substitute whole blood) is as follows:
[0054] Silicon dioxide with a diameter of 6 μm and a simulated liquid with a volume ratio of 30:100, the simulated liquid includes 30 wt% bovine serum albumin, 3 wt% myoglobin and 1 wt% lysozyme, and the balance is water;
[0055] Whole bovine blood was used as the control group.
[0056] The present invention adopts figure 1The shown monitoring device changes with time the transmembrane pressure of whole blood simulated liquid and bovine whole blood, and the test data are shown in Table 1;
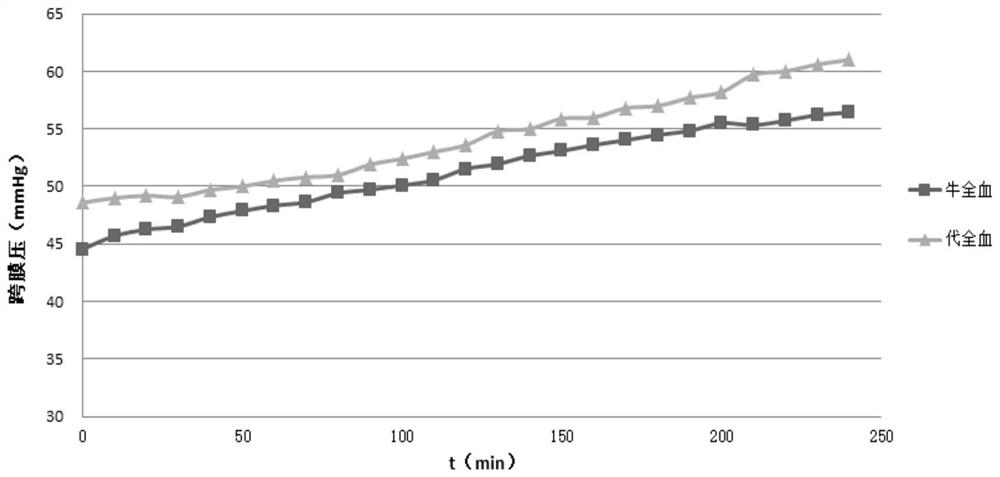
[0057] The transmembrane pressure of the whole blood simulated liquid of table 2 embodiment 2 changing over time
[0058] time / min Transmembrane pressure of bovine whole blood (mmHg) The transmembrane pressure (mmHg) of embodiment 2 0 44.6 48.6 10 45.7 49.0 20 46.3 49.2 30 46.5 49.1 40 47.4 49.7 50 47.9 50.0 60 48.3 50.5 70 48.6
Embodiment 3
[0061] The composition of the whole blood simulated liquid (substitute whole blood) is as follows:
[0062] Polystyrene microspheres with a diameter of 6 μm and a simulated liquid with a volume ratio of 28:100, the simulated liquid includes 10wt% dextran with a molecular weight of 70kDa, 15wt% albumin and 1wt% lysozyme, and the balance is water;
[0063] Whole bovine blood was used as the control group.
[0064] The present invention adopts figure 1 The shown monitoring device changes with time the transmembrane pressure of whole blood simulated liquid and bovine whole blood, and the test data are shown in Table 3;
[0065] The transmembrane pressure of the whole blood simulated liquid of table 3 embodiment 3 changes over time
[0066] time / min Transmembrane pressure of bovine whole blood (mmHg) The transmembrane pressure (mmHg) of embodiment 3 0 44.6 44.0 10 45.7 44.5 20 46.3 45.0 30 46.5 45.7 40 47.4 46.0 50 47.9 46.2 60
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap