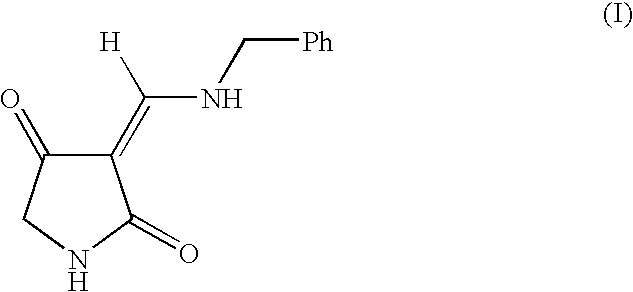
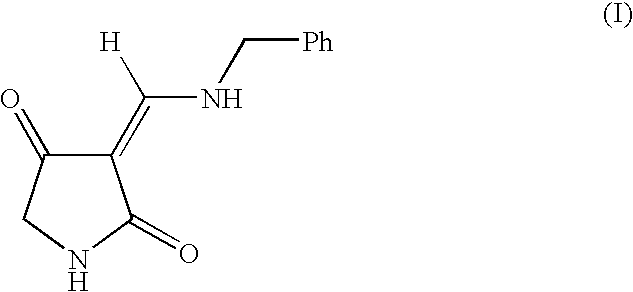
Treatment of CNS and pain disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0078]Hot plate test. Male mice weighing from 25 to 30 g were used in this test. The thermal technique (hot plate analgesia meter) was used to evaluate analgesic effect after the injection of drugs or saline. The surface of the plate was maintained at a temperature of 55.0±0.5° C. Analgesic effect was determined 30 min. after compound injection. Time (in seconds) to either hind paw licking or jumping was recorded as pain response latency. To avoid the likelihood of tolerance of mice to hot plate, all mice were exposed to hot plate only once through the whole experiment. The mice were exposed to hot plate only once; either before or after injection of drug.
[0079]In this hot plate test the tetramic derivative of the invention exhibits an analgesic effect after peroral administration in mice at a dose of 50 mg / kg. A similar effect was observed with phenytoin and valproate at 100 and 500 mg / kg respectively.
example 2
[0080]Tail-flick test. The tail-flick test, using a radiant heat source was carried out to study antinociception. Each mouse was placed in an acrylic tube and its tail was laid across a Nichrome™ wire coil, which was heated by the passage of an electric current. The intensity of the current was kept at 5 mA. All animals were screened for the tail-flick response. Only those showing a flick within 4 sec were included in the study. After 1 h of final dosing, the mice were submitted to three tail-flick tests.
[0081]In this tail-flick test the tetramic derivative of the invention exhibits an analgesic effect after peroral administration.
[0082]At the highest doses tested (500 mg / kg), the tetramic derivative of the invention exhibits a relatively better analgesic effect compared to phenytoin and valproate (100 and 500 mg / kg respectively). All data were compared with the same control group.
example 3
[0083]Formalin paw test. The mice received an injection of formalin (4.5%, 20 μl) to the left hind paw. The irritation caused by the formalin injection elicits a characteristic biphasic behavioral response as quantified by the amount of time spent licking the injured paw. The first phase (˜5-10 minutes) represents direct chemical irrigation, and the second phase (˜20-30 minutes) is thought to represent pain of neuropathic origin (Tjølsen A et al. Pain (1992) 51:5-17). The two phases are separated by a quiescent period in which behavior returns to normal. The effectiveness of test compounds to reduce the painful stimuli is assessed by counting the amount of time spent licking the injured paw in the two phases.
[0084]In this inhibition of pain behavior test, the tetramic derivative of the invention displays a 5% reduction in pain score in the chronic phase at a dose of 30 mg / kg. Similar reduction was seen with gabapentin and morphine at 20 mg / kg and 1.25 mg / kg, respectively, compare
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
| Disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap