Alkylphosphorofluoridothioates having low wear volume and methods for synthesizing and using same
a technology of alkylphosphorofluoridothioates and wear volume, which is applied in the field of lubricants, can solve the problems of increasing automotive emissions, affecting the yield of lubricants, and many lubricants currently in use that have undesirable side effects, and achieves the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method for Making Alkylphosphorofluoridothioates
[0033]General Experimental Details
[0034]All of the reagents were purchased from commercial suppliers and were used without purification unless otherwise specified. Reactions involving air- or water-sensitive compounds were conducted in oven-dried (overnight) glassware under an atmosphere of dry argon. Anhydrous solvents were freshly prepared according to well documented methods. NMR spectra (1H, 13C, 31P, and 19F) were recorded on JEOL eclipse+ instrument at 500, 125, 202 and 470 MHz respectively using CDCl3 as solvent and TMS as reference unless otherwise noted. Melting points were obtained in capillary tubes on a MeI-Temp II apparatus, and the thermometer was uncorrected. Infrared (IR) spectra were obtained on a Bruker Vector 22 FT-IR spectrometer, using KBr pressed pellets for solids or neat films between KBr plates for liquids and oils, and are reported in cm−1 with a resolution of 4 cm−1. High resolution electrospray ionization time-
example 2
Tribological Testing of Alkylphosphorofluoridothioates
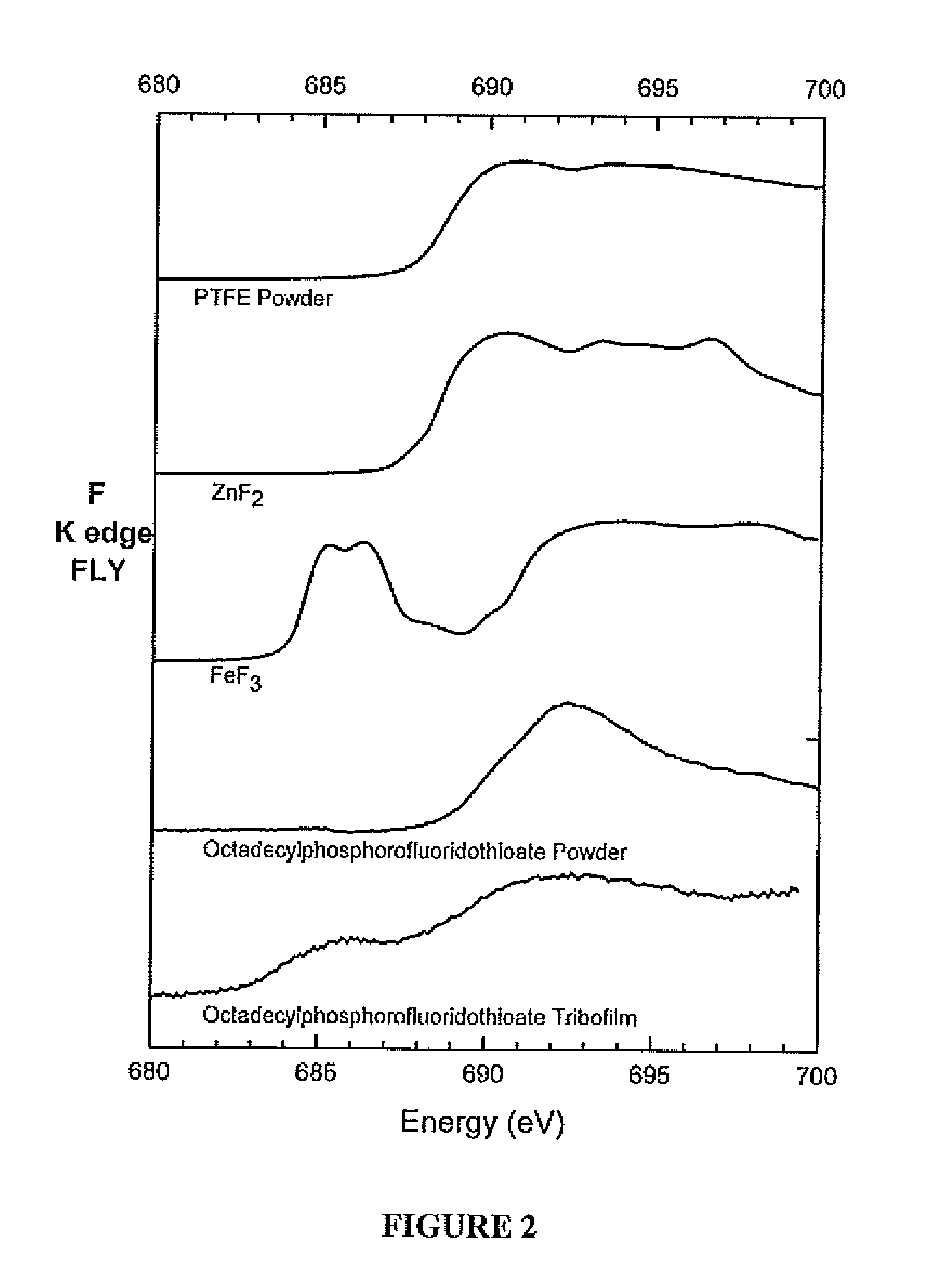
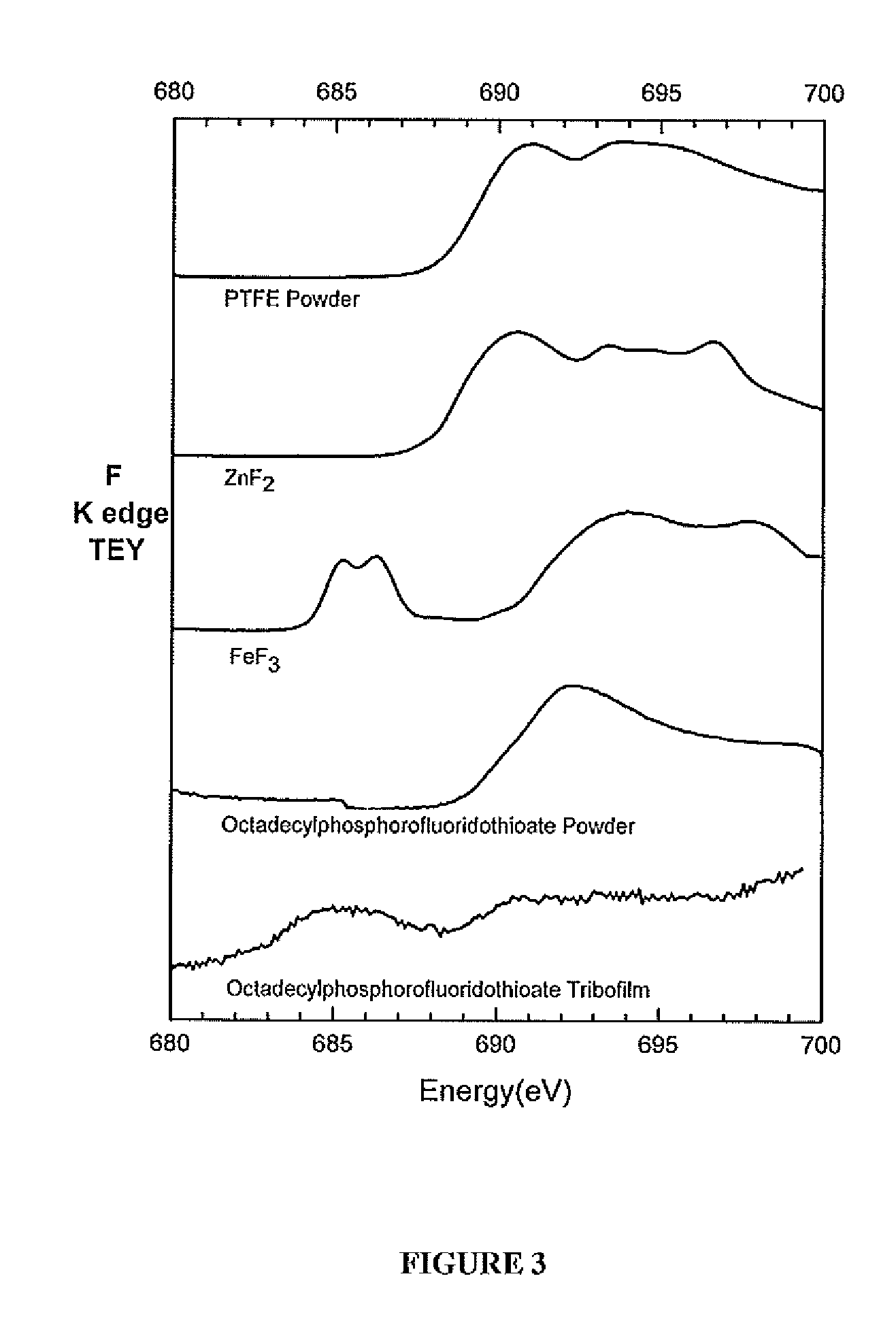
[0068]All compounds were diluted in 100 N base oil to a phosphorus concentration of 0.1 wt % and tribological tests were conducted in a High Frequency Reciprocating Ball on Stationary Flat tribometer. The ball and the flat were both made of 52100 steel and were immersed in the oil mixture. The test temperature was 100° C., the load applied on the ball was 1.0 Kg, the frequency of the test was 50 Hz, and the duration of the test was 1 hour. Wear volume was measured by Vecco Wyko NT9100 Optical Profiler System with Vision®. XANES (X-ray Absorption Near Edge Structure) was obtained at Canadian Light Source, Saskatoon, SK, Canada. After the completion of the test, the flat sample was cleaned and the amount of material that was removed by wear was measured using an optical profilometer.
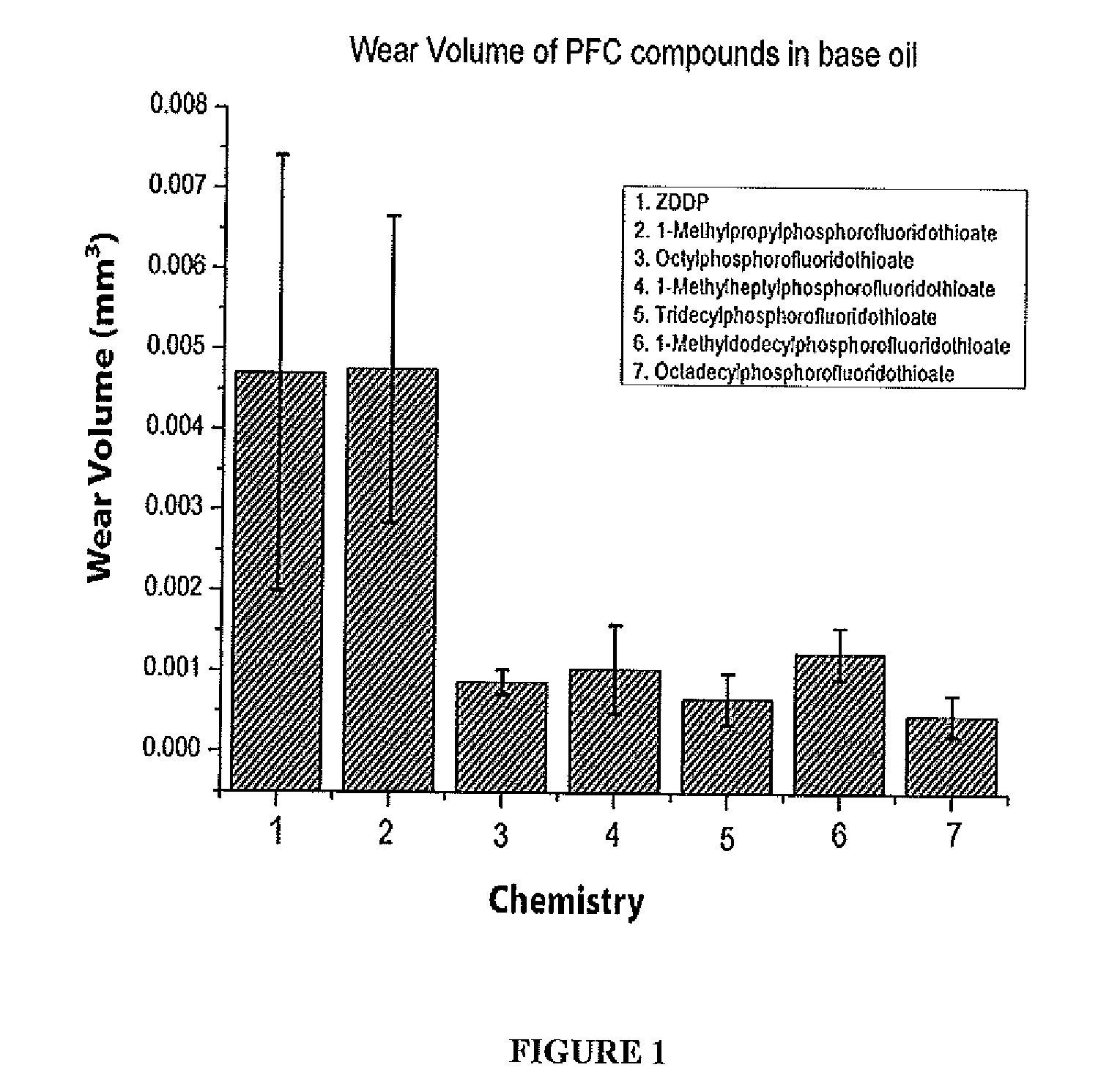
[0069]Wear Volume Results
[0070]FIG. 1 shows the wear volume for the compounds shown in Table 1, including ZDDP for reference in lane 1. The compound illus
example 3
Thermal Stability
[0078]Thermal stability of the alkylphosphorofluoridothioates was examined using a Thermogravimetric Analyzer (TGA) to examine the decomposition temperature. FIG. 9 shows the change in weight of different alkylphosphorofluoridothioates as a function of temperature when they were heated in the TGA in an environment of argon at a heating rate of 5° C. per minute. The alkylphosphorofluoridothioates made with primary alcohols are more stable than the ones made from secondary alcohols. In addition, the alkylphosphorofluoridothioates with longer chain alkyl groups are more stable than ones with shorter chain alkyl groups.
[0079]The various thermal stabilities of the compounds mean that there is quite a bit of capability to tailor the operative temperature range in which wear protection can be achieved as well as length of time for the wear protection (time-temperature profiles for wear protection). This flexibility comes from both the chemical structure of the compounds as we
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap