Organoaminosilane Precursors and Methods for Depositing Films Comprising Same
a technology of organic aminosilane and precursors, applied in the field of organic aminosilane precursors, can solve problems such as handling and usage problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Phenylmethylaminosilane Using Silyl Exchange Reaction
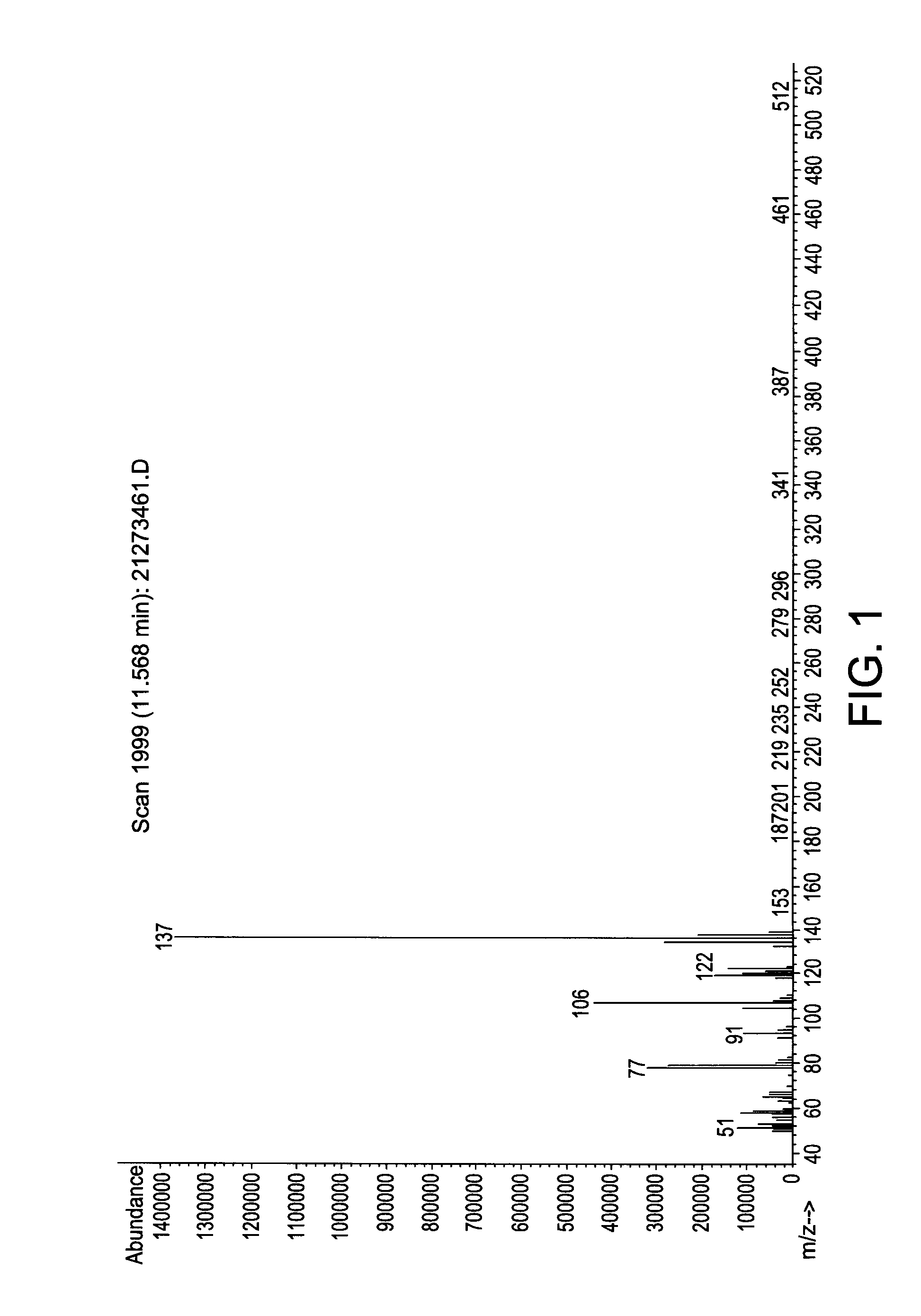
[0113]In a 500 ml Schlenk flask, 64.2 grams (g) (0.6 mol) N-methylaniline and 131 g (1.0 mol) di-isopropylaminosilane were stirred at ambient temperature under a nitrogen atmosphere for 24 hours. The relatively lower boiling point by-product di-isopropylamine was removed with vacuum at a pressure of 20 mmHg and room temperature (25° C.). The reaction mixture was stirred for another 24 hours. The end-product phenylmethylaminosilane (73.6 g, 89.5% yield) was obtained by vacuum distillation with a boiling point of 60° C. at 5 mm Hg. The end-product was characterized by mass spectroscopy (MS) which is provided in FIG. 1 and shows, among other things, peaks at 137, 122, 106, 91, and 77. The molecular weight of the phenylmethylaminosilane was 137.27.
example 2
Alternative Synthesis Method for Phenylmethylaminosilane Using Monochloroaminosilane as a Reagent
[0114]In a 2000 ml three-necked flask equipped with a mechanical stirrer, a condenser, and a gas bubbling inlet, 1000 ml hexane, 53.5 g (0.5 mol) N-methylaniline, and 50.5 g (0.5 mol) triethylamine were cooled to −20° C. with stirring under nitrogen atmosphere. Monochlorosilane (MCS) was bubbled through the reaction mixture. A white solid precipitate was formed. After the reaction was complete, the temperature of the reaction mixture was allowed to warm to room temperature while stirring continued for an additional 2 hours at room temperature. Solid triethylamine hydrochloride salt was removed by filtration, and the solvent hexane was removed by distillation. The product phenylmethylaminosilane (51.3 g, 75% yield) was obtained by vacuum distillation with boiling point of 60 C at 5 mm Hg. The compound was characterized by mass spectroscopy and confirms that the product is phenylmethylaminosi
example 3
Synthesis of Phenylethylaminosilane Using Silyl Exchange Reaction
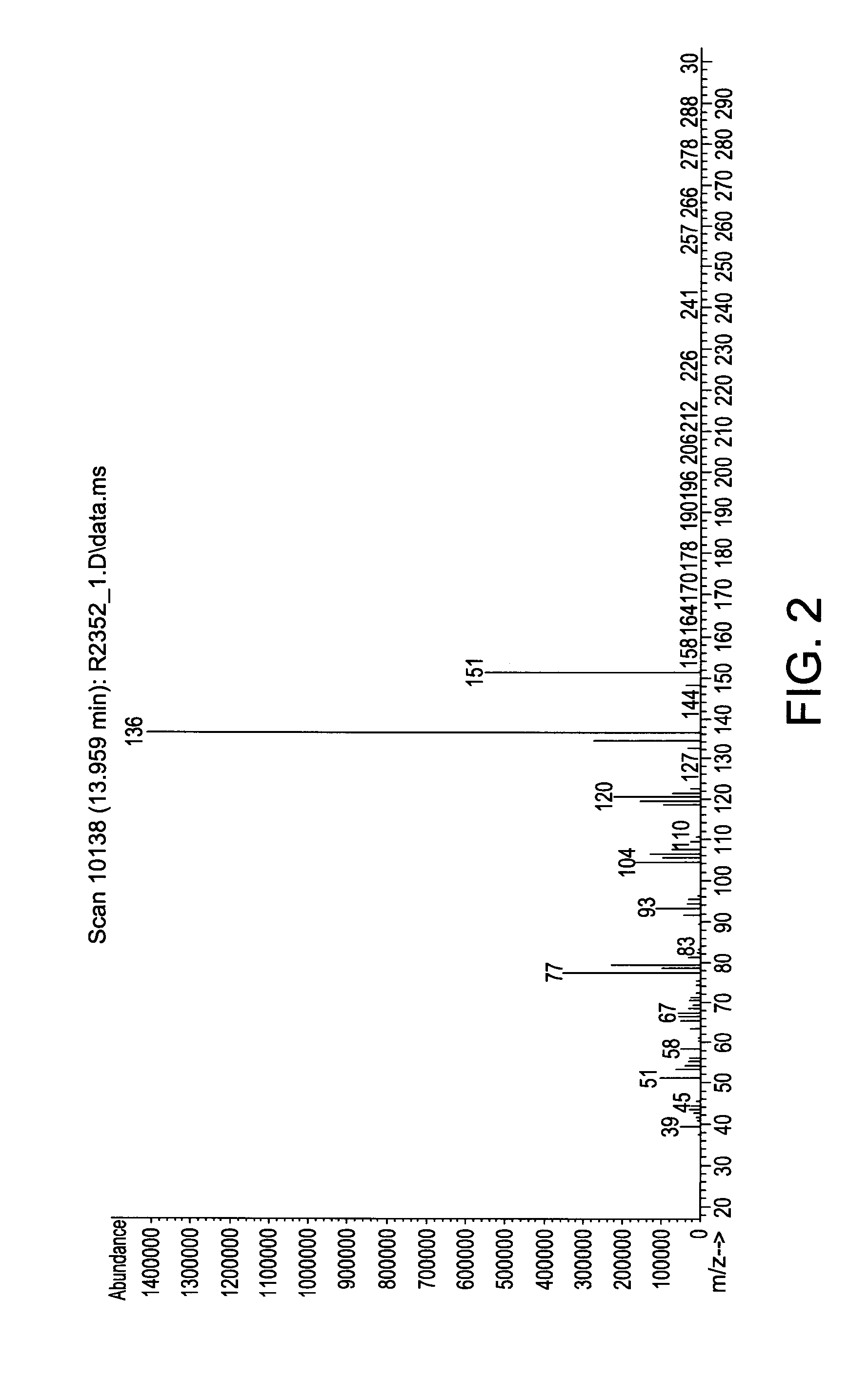
[0115]In a 500 ml Schlenk flask, 60.5 g (0.5 mol) N-ethylaniline and 131 g (1.0 mol) di-isopropylaminosilane were stirred at ambient temperature under a nitrogen atmosphere for 24 hours. The relatively lower boiling point by-product di-isopropylamine was removed with vacuum at a pressure of 20 mmHg and room temperature (25° C.). The reaction mixture was stirred for another 24 hours. The end-product phenylethylaminosilane was obtained by vacuum distillation. The end-product was characterized by mass spectroscopy (MS) which is provided in FIG. 2 and shows, among other things, peaks at 151, 150, 136, 120, 106, 93, and 77. The molecular weight of the phenylethylaminosilane was 151.28.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap