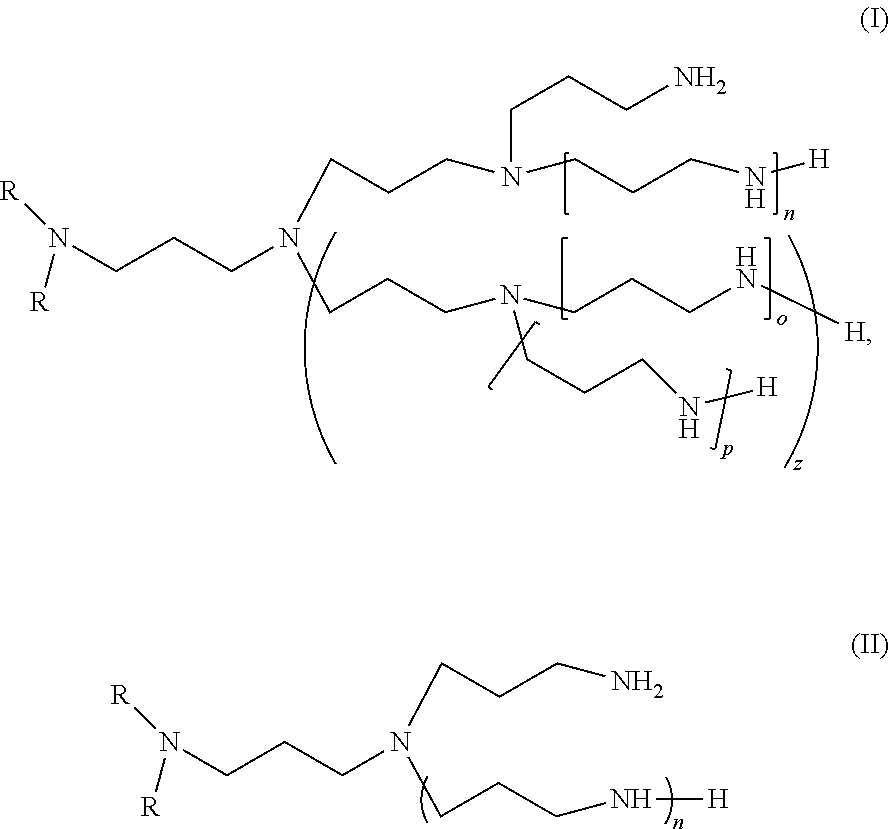
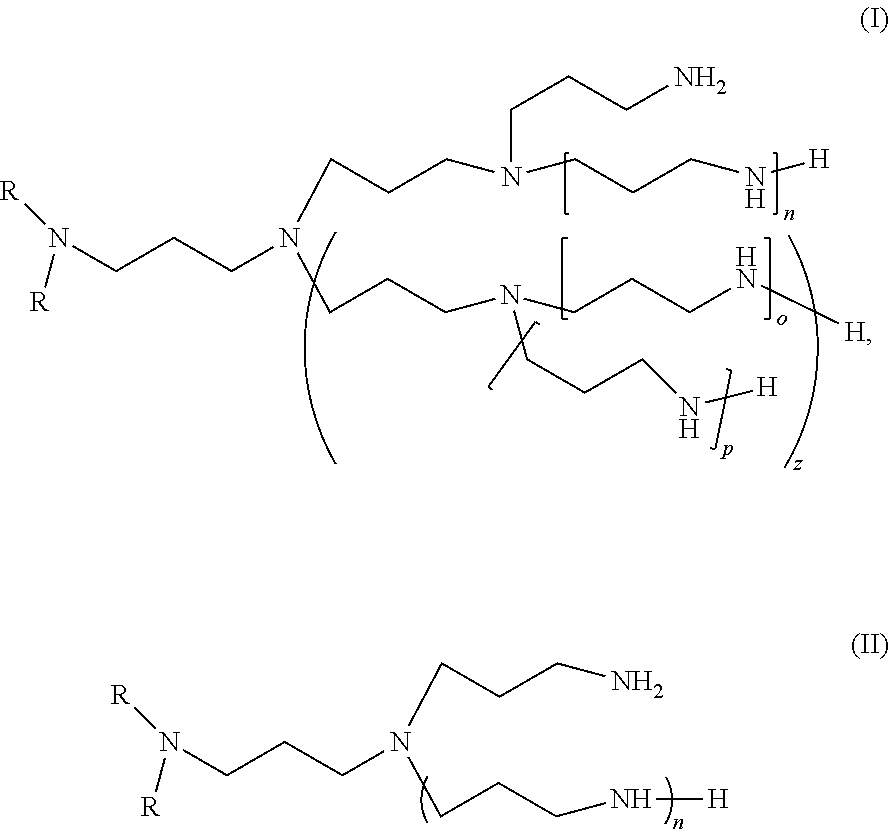
Dialkyl-polyalkylamine compositions, process for their preparation and their use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032]A fully branched product with 4 amine functions was prepared using a 1 L glass reactor with turbine stirrer to which chemicals can be dosed using a Prominent Gamma / L membrane pump and which was thermostatted using a Lauda K6KP heating bath.
Raw Materials
[0033]
IntakeMolw.IntakeChemicalSupplier(g)(g / mol)(mol)Duomeen 2HTAkzoNobel342.05660.604Hydrochloric acid (36%)J T Baker3.0636.50.014WaterTap1.1218.00.062IsopropanolJ T Baker34.260.10.569Sodium carbonateAcrosAs needed, see textAcrylonitrileAcros81.553.11.299Raney CobaltCatAlloyAs needed, see textAmmoniaAir productsAs needed, see text
Procedure & Results
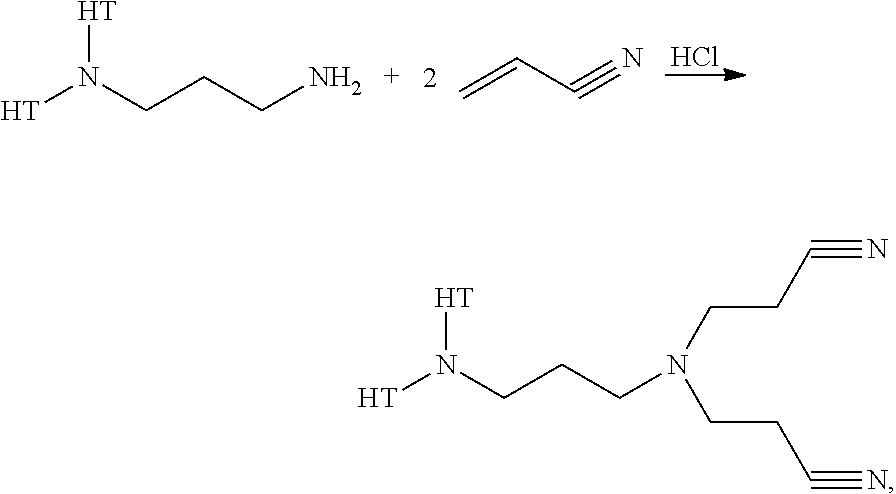
[0034]The cyano-ethylation step is performed by charging the reactor with Duomeen 2HT, isopropanol (co-catalyst and solvent for the dicyano-product that is formed), water, and HCl, and subsequent dosing, in approximately three hours, of the acrylonitrile. Reaction pathway:
wherein HT stands for hydrogenated tallow.
[0035]After a conversion of 80% the reaction rate was so slow that th
example 2a and 2b
[0038]A mixture of linear and branched product (Tetrameen 2HTb) was prepared by the two cycle procedure wherein the cyano-ethylation and the hydrogenation steps above were repeated. The 0.6 mole of the Duomeen 2HT was combined with 0.65 mole of acrylonitrile and reacted in the first cyano-ethylation step. After hydrogenation, the triamine was combined with another 0.65 mole of acrylonitrile and reacted. At the end of each cyano-ethylation step, NMR was used to analyze the reaction mixture and to determine if one mole of acrylonitrile had reacted per mole of starting material. If the reaction was found to be too low, some additional acrylonitrile was dosed and after 1 hour the analysis was repeated. This cycle was repeated till the desired reaction was obtained. The final product was analyzed using GC-MS applying the following conditions
Gas chromatographTRACE ULTRA GC InterscienceMS systemISQ GC-MSColumnFused silica WCOT, 20 m × 0.32 mm IDstationary phaseSil 5 CB, 100% polydimethyl-silo
examples 3-5
[0041]To a commercial lubricant (Talusia HR70, which is overbased and comprises CaCO3, from Total LubeMarine), 5 wt % of the 2HTb of example 2a, 2HTY, or a 50 / 50 blend of 2HTY and 2HTb was added and thoroughly blended, followed by neutralization of 50 BN points of the lubricant composition with 95% sulfuric acid, in order to simulate the phenomenon of neutralization of the composition to be closer to real conditions of use of the lubricating composition in a marine engine.
[0042]In this process the amines together with the overbased detergents neutralizes the sulfuric acid. The generated sulfate ions become the counterions of the positively charged ammonium groups and / or react with the calcium of the CaCO3 to form gypsum, CaSO4.
[0043]Measurements of the viscosity (Pa·s) at 40° C. of the three acidized blends of lubricant and alkylpolyamines, as prepared above, were performed by measuring the viscosity at a shear rate of 0.05 s−1 as displayed in the tables. All measurements were perf
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap