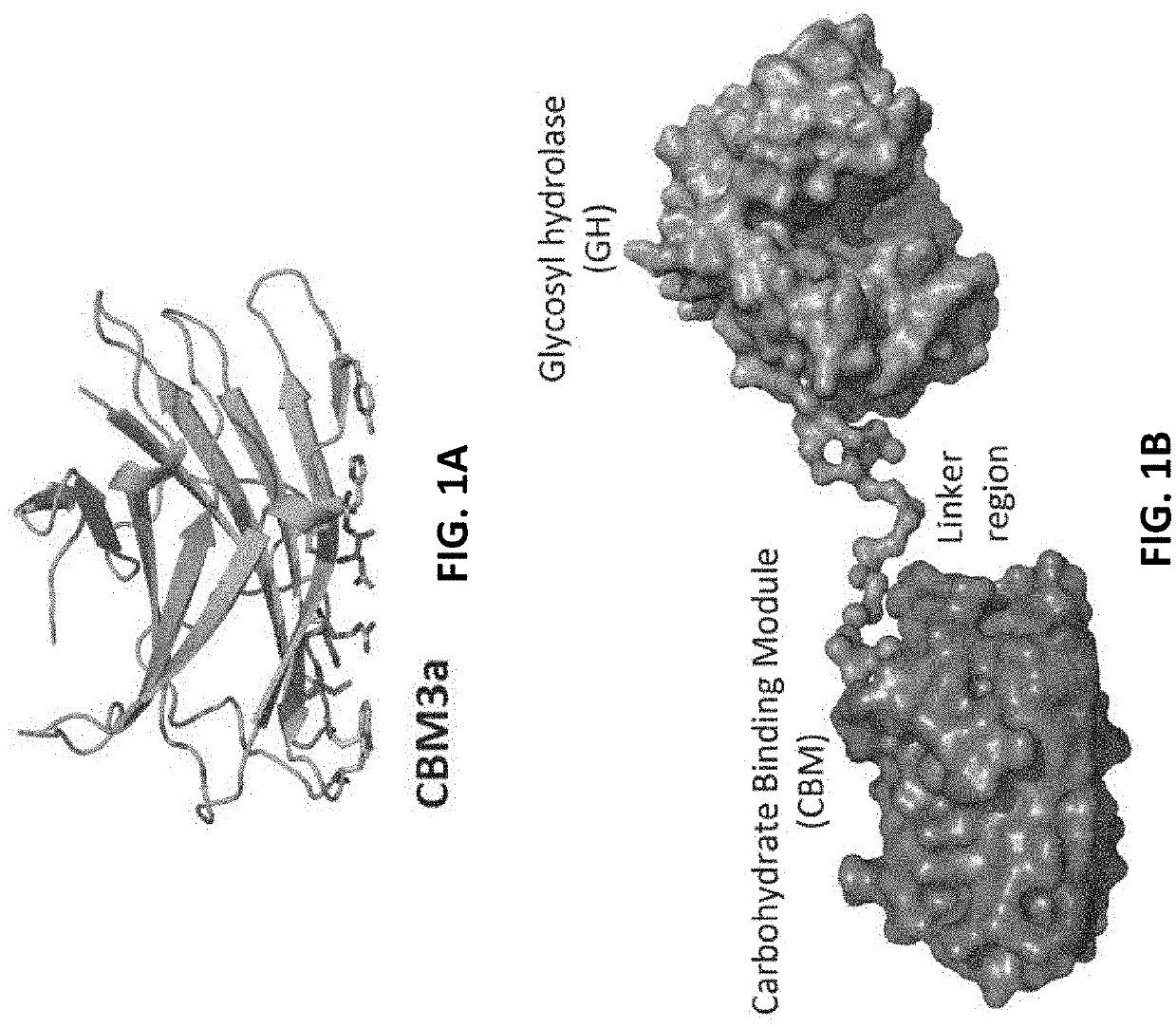
Engineered Carbohydrate-Active Enzymes for Glycan Polymers Synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
[0073]The invention is further described in detail by reference to the following experimental examples. These examples are provided for purposes of illustration only, and are not intended to be limiting unless otherwise specified. Thus, the invention should in no way be construed as being limited to the following examples, but rather, should be construed to encompass any and all variations which become evident as a result of the teaching provided herein.
[0074]Without further description, it is believed that one of ordinary skill in the art can, using the preceding description and the following illustrative examples, practice the claimed methods of the present invention. The following working examples therefore, specifically point out the preferred embodiments of the present invention, and are not to be construed as limiting in any way the remainder of the disclosure.
Materials and Methods
Generating DNA Constructs Via Site-Directed Mutagenesis
[0075]Purification of wt DNA from E. coli
[00
example 1
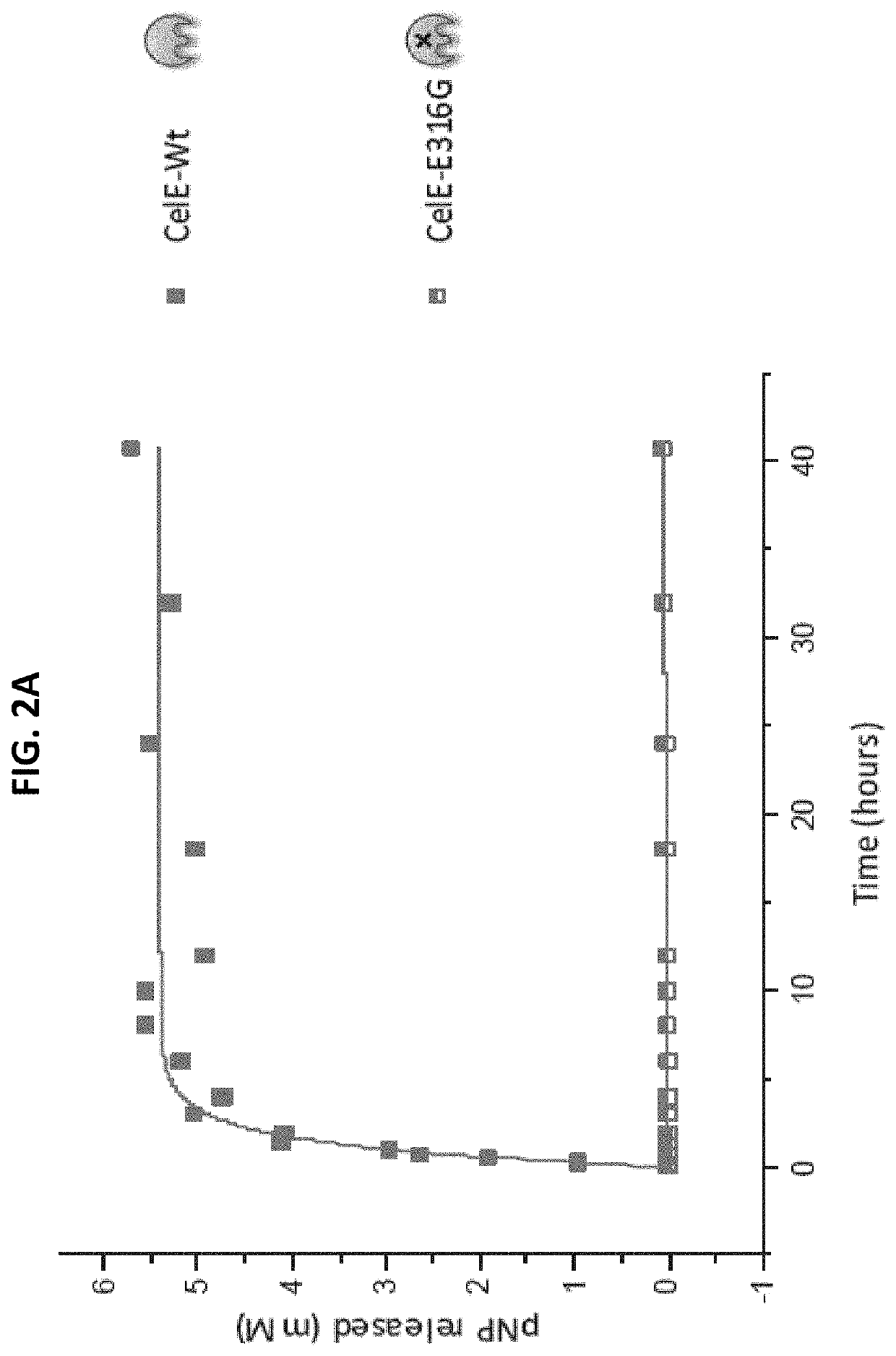
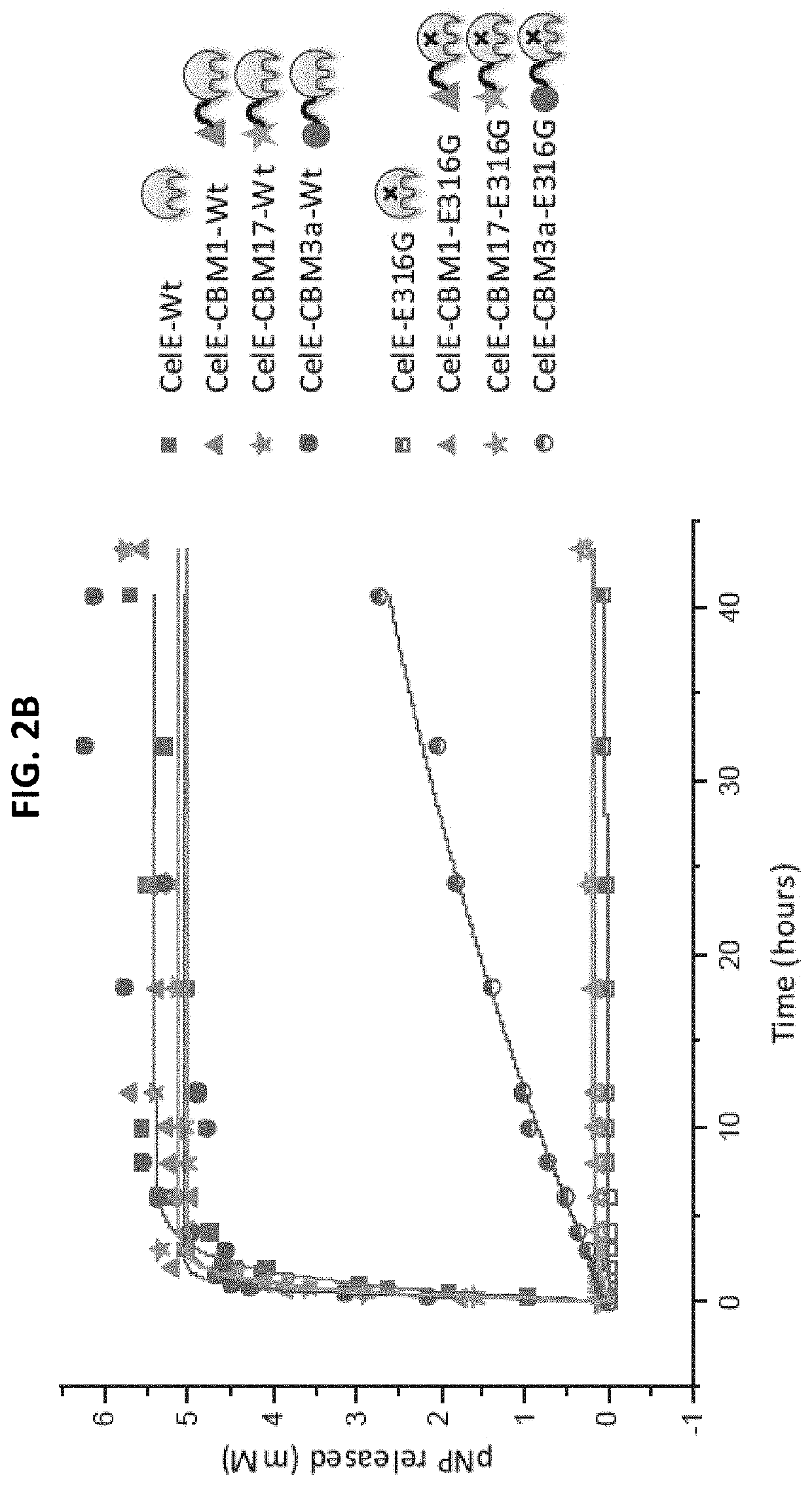
[0085]The effect of each CBM on the transglycosylation activity of CelE was tested by reacting 2 nanomoles of each construct with 10 mM of pNP-Cellobiose. The reaction conditions were 60° C., pH 6.5 and an RPM of 350. The reaction was stopped after 7 hours by freezing the samples at −20° C. The results of these reactions are as follows.
[0086]The products of the pNP-Cellobiose reactions were analyzed in two different ways. First, the pNP-C conversion was measured by measuring the pNP absorbance of each sample. The pNP absorbance was measured by mixing 10 μL of sample with 90 μL of deionized water and 100 μL of 0.1 M NaOH and the comparing that value to the standard curve produced by pNP samples of known concentration. The standard curve of the pNP samples and the pNP-Cellobiose conversion can be seen below in FIGS. 3a and 3b, respectively.
[0087]The samples with the highest percent conversion of pNP-Cellobiose showed the higher activity. These results show that CBMs can have a positive e
example 2
[0091]The effect of varying linker length on the transglycosylation activity of CelE was tested by reacting different construct with pNP-Cellobiose at 60° C. for 4 hours.
[0092]FIGS. 7a and 7b show that shortening the length of the linker domain from ˜40 amino acids to 6 amino acids for CelE-E316G-CBM3a results in a significant drop in transglycosylation reaction. These results provide supporting evidence that the CBM domain is interacting with the mutant catalytic domain to increase biosynthesis capability of the enzyme construct.
Enumerated Embodiments
[0093]The following exemplary embodiments are provided, the numbering of which is not to be construed as designating levels of importance.
[0094]Embodiment 1 provides a construct comprising a Glycoside Hydrolase (GH) catalytic domain (CD), which is conjugated to a carbohydrate binding module 3a (CBM3a), wherein the CD is mutated with respect to its wild-type form so that the mutated CD is capable of catalyzing glycan polymer synthesis.
[009
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Catalytic activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap