Methods for the re-derivation of diverse pluripotent stem cell-derived brown fat cells
a technology of brown fat cells and pluripotent stem cells, applied in the field of stem cell biology, can solve problems such as increasing age-dependent risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Effects of Adipocyte Differentiation Conditions Diverse Clonal Embryonic Progenitor Cell Lines
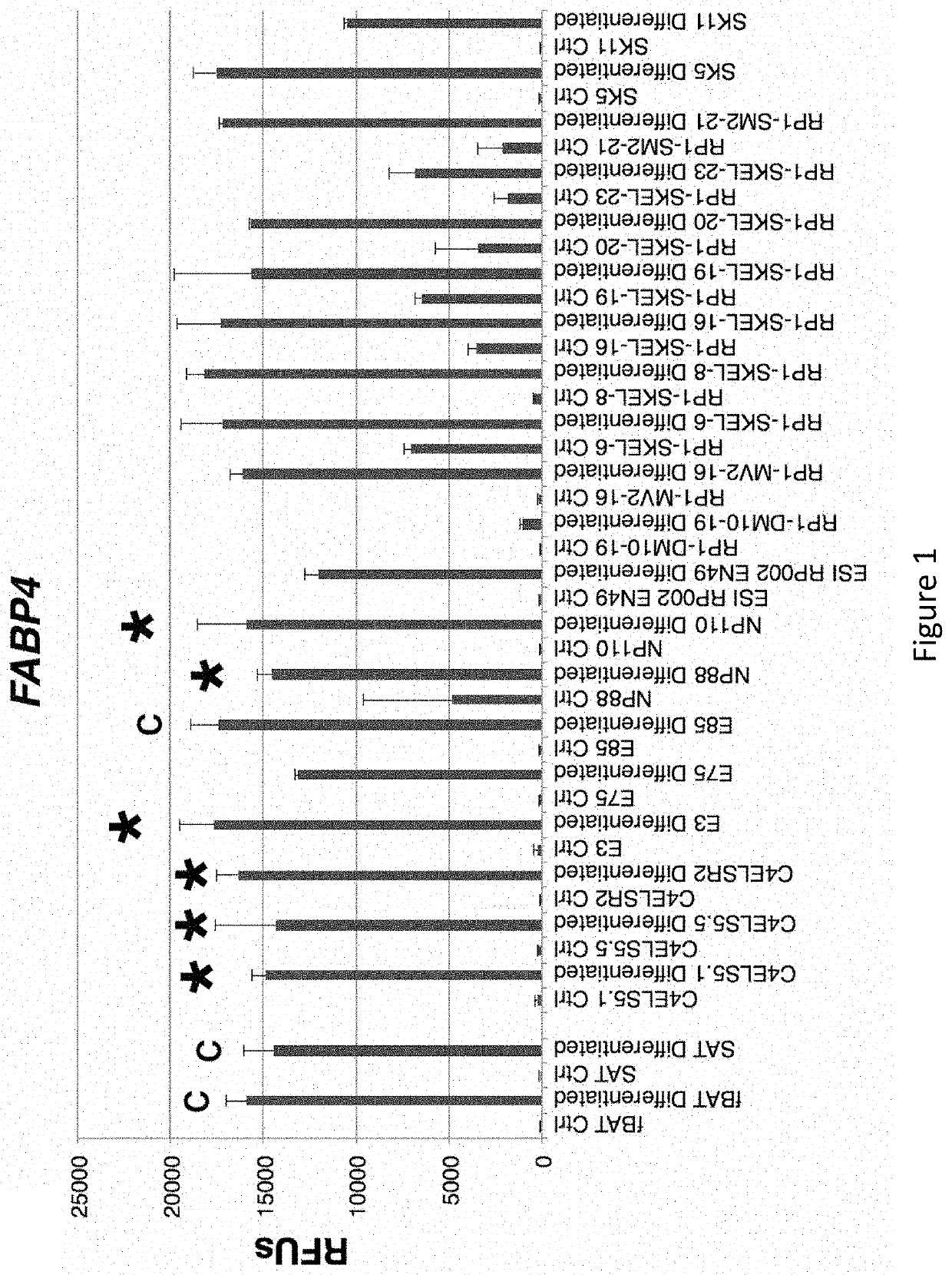
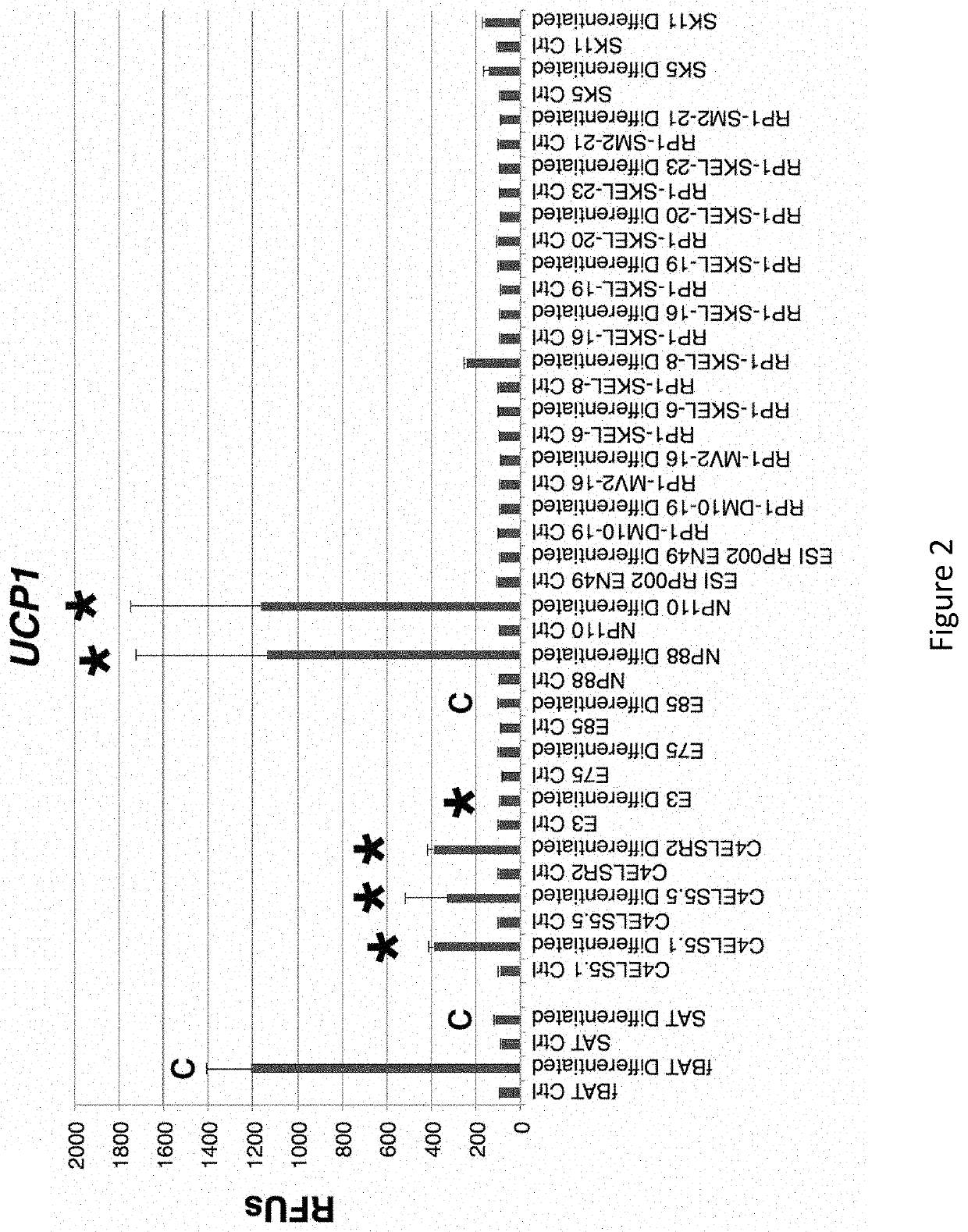
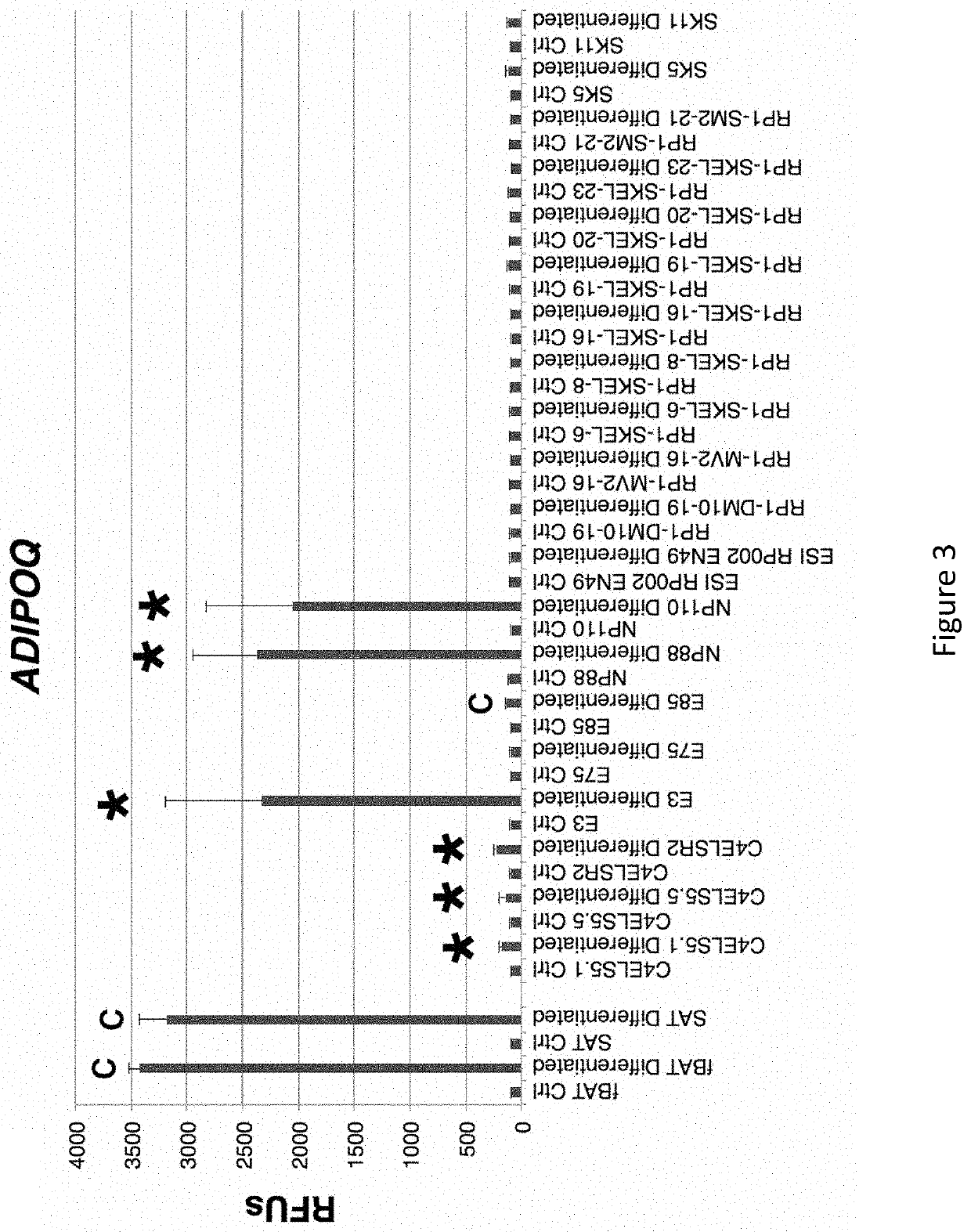
[0320]As shown in FIG. 1, many diverse clonal embryonic progenitor cell lines (Ctrl) expressed increased levels of the adipocyte marker FABP4 when cultured in HyStem beads supplemented with 10 ng / ml BMP4, 1.0 μM rosiglitazone, 2.0 nM triiodothyronine (T3), and for the last 4 hours prior to use, 10 μM CL316243 (Differentiated). As shown in FIGS. 2-4, only a subset of these diverse clonal hEP cell lines exhibited an up-regulation of UCP1, BETATROPHIN (also known as C19ORF80, LOC55908, and C19orf80) or ADIPOQ. While cultured fetal brown fat tissue (fBAT)-derived preadipocytes and subcutaneous adipose tissue (SAT)-derived preadipocytes showed only a minority of cells staining with the adipocyte marker Oil Red-O as described herein, and a minority of cells staining for UCP1, essentially all cells stained for the markers in differentiated cultures of the NP88 and NP110 lines (FIG. 5). As shown
example 2
Manufactured from Universal Donor cGMP Human ES Cell Lines
[0321]Clinical grade cGMP-compatible human ES cell lines are genetically modified to constitutively express CTLA4-Ig and PD-L1. (Z. Rong, et al, An Effective Approach to Prevent Immune Rejection of Human ESC-Derived Allografts, Cell Stem Cell, 14: 121-1.30 (2014) incorporated herein by reference. In brief, the human ES cell lines described by J. Crook et al, The Generation of Six Clinical-Grade Human Embryonic Stem Cell Lines, Cell Stem Cell 1, November 2007, are genetically-modified to constitutively express the genes CTLA4-Ig and PDL1 using a BAC-based targeting vector such as the HPRT BAC clone RP11-671P4 (Invitrogen) and the targeting vector is constructed using recombineering as described (Rong et al, A scalable approach to prevent teratoma formation of human embryonic stem cells, J. Biol. Chem. 287: 32338-32345; Song et al, Modeling disease in human ESCs using an efficient BAC-based homologous recombination system, Cell St
example 3
ization of Additional Diverse Embryonic BAT Cell Progenitors Derived from hESCs
[0323]A number of specific human ES cell-derived clonal embryonic progenitor cell lines are disclosed for the first time herein. Specifically, these cell lines have been given the following designations: NP88, NPCC SM19, NPCC SM23, NPCC SM28, NPCC SM31, NPCC SM36, NP111 SM, NP77 EN, NP78 EN, NP80 EN, NP91 SM, NP92 SM, NP93 SM, NP85 EN, NP113 SM, NPCC SM27, and SK1 All clonal embryonic progenitor cell lines were derived from the pluripotent cell stem cell line Envy (Costa et al., The hESC line Envy expresses high levels of GFP in all differentiated progeny, NAT Methods 2(4): 259-260 (2005)) with the exception of line SK1 which was derived from hESC cell line H9 (WA09) using methods described under the heading “Novel Uses of Cells With Prenatal Patterns of Gene Expression”; U.S. patent application Ser. No. 11 / 504,047 filed on Nov. 21, 2006 and entitled “Methods to Accelerate the Isolation of Novel Cell Strai
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap