Method for improving in-vitro expression of cytochrome P450 enzyme family
An in vitro expression, cytochrome technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, plant genetic improvement, etc., can solve the problems of low yield and difficult to meet research needs, and achieve considerable economic benefits and a good society. effect of benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0032] Example 1
[0033] 1. Construction and identification of recombinant transfer vector pFastBac1-CYP2A13 plasmid
[0034] pFastBac1-CYP2A13 is kept in this laboratory. After screening on ampicillin resistance plates, Escherichia coli containing recombinant plasmids was obtained, and bacterial liquid DNA was extracted to obtain recombinant transfer vector plasmid DNA. Use double enzyme digestion to verify pFastBac1-CYP2A13.
[0035] 2. Construction and identification of recombinant eukaryotic expression virus Ac-Bacmid-CYP2A13 DNA
[0036] The constructed pFastBac1-CYP 2A13 recombinant plasmid was transformed into competent cells of DH10Bac Escherichia coli. With the help of the helper plasmid of DH10Bac Escherichia coli, it was transposed by Tn7 transposon, and the gene fragments of each CYP2A13 were inserted into the shuttle vector Bacmid. The kanamycin, gentamicin and tetracycline tertiary antibodies were screened and blue-white spot screened to obtain recombinant Ac-Bacmid-CYP
Example Embodiment
[0042] Example 2
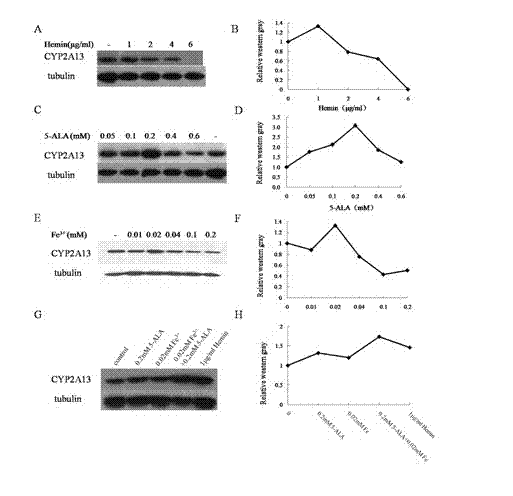
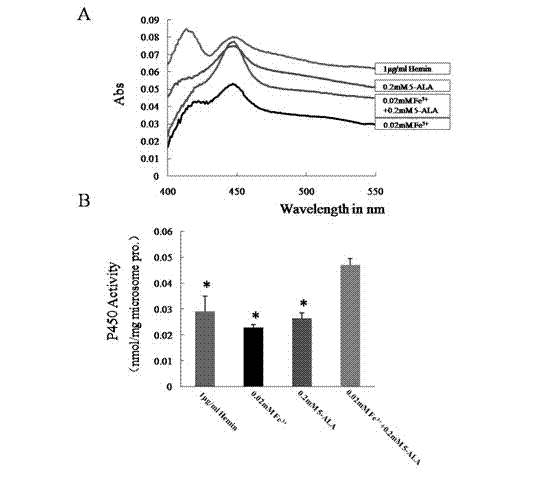
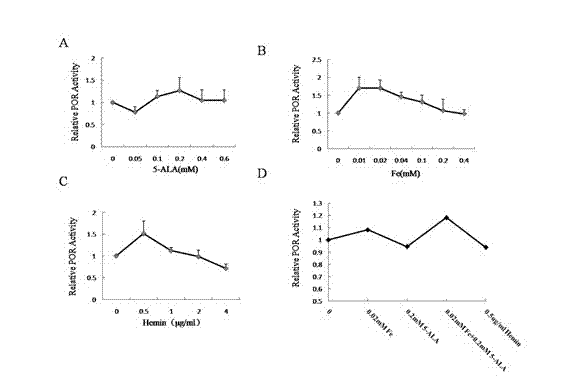
[0043] Compared with Example 1, it is the same except that the cofactor in step 4 is 0.05-0.6mM 5-ALA.
Example Embodiment
[0044] Example 3
[0045] Compared with Example 1, except that the cofactor in step 4 is 0.01-0.4mM Fe 3+ Except for the difference, everything else is the same.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap