Modified lithium-rich manganese-based cathode material for lithium ion battery
A lithium-ion battery, lithium-rich manganese-based technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of low initial Coulombic efficiency, limited application, poor rate performance, etc., and achieve improved cycle performance, low price, and improved The effect of the first Coulombic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (La 0.85 Sr 0.15 ) 0.9 MnO 3-δ Solution coated Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2
[0024] The coating content is 1wt% (La 0.85 Sr 0.15 ) 0.9 MnO 3-δ Take La(NO 3 ) 3 ·6H 2 O, Sr(NO 3 ) 3 , Manganese acetate is used as the coating raw material, and it is formulated into a clear solution with citrate according to the stoichiometric ratio, and the ratio of metal ions to citric acid is 1:1. Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 As the coating object, take 2g Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 Add the above solution, after ultrasonic dispersion, stir and heat to gel, dry the gel at 80°C for 5h, and treat at 850°C for 10h to obtain a certain amount of coated Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 . Li with a mass ratio of 80:10:10 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 The sample, conductive carbon black and polyvinylidene fluoride were evenly mixed to make a working electrode, and the negative electrode was a metal lithium sheet. The irreversible capaci
Embodiment 2
[0026] (La 0.85 Sr 0.15 ) 0.9 MnO 3-δ Solution coated Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2
[0027] Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 As a coating object, the coating content is 3wt% (La 0.85 Sr 0.15 )- 0.9 MnO 3- δ Take La(NO 3 ) 3 ·6H 2 O, Sr(NO3 ) 3 , manganese acetate as the coating raw material, a certain amount of citrate as a complexing agent, after drying at 80 ℃, calcined at 850 ℃ / 10h. Li with a mass ratio of 80:10:10 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 The sample, conductive carbon black and polyvinylidene fluoride were uniformly mixed to make a working electrode, and the negative electrode was prepared by buckling metal lithium sheet for testing. The same irreversible capacity loss decreased significantly.
Embodiment 3
[0029] (La 0.85 Sr 0.15 ) 0.9 MnO 3-δ Powder Mechanical Mixing and Coating Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2
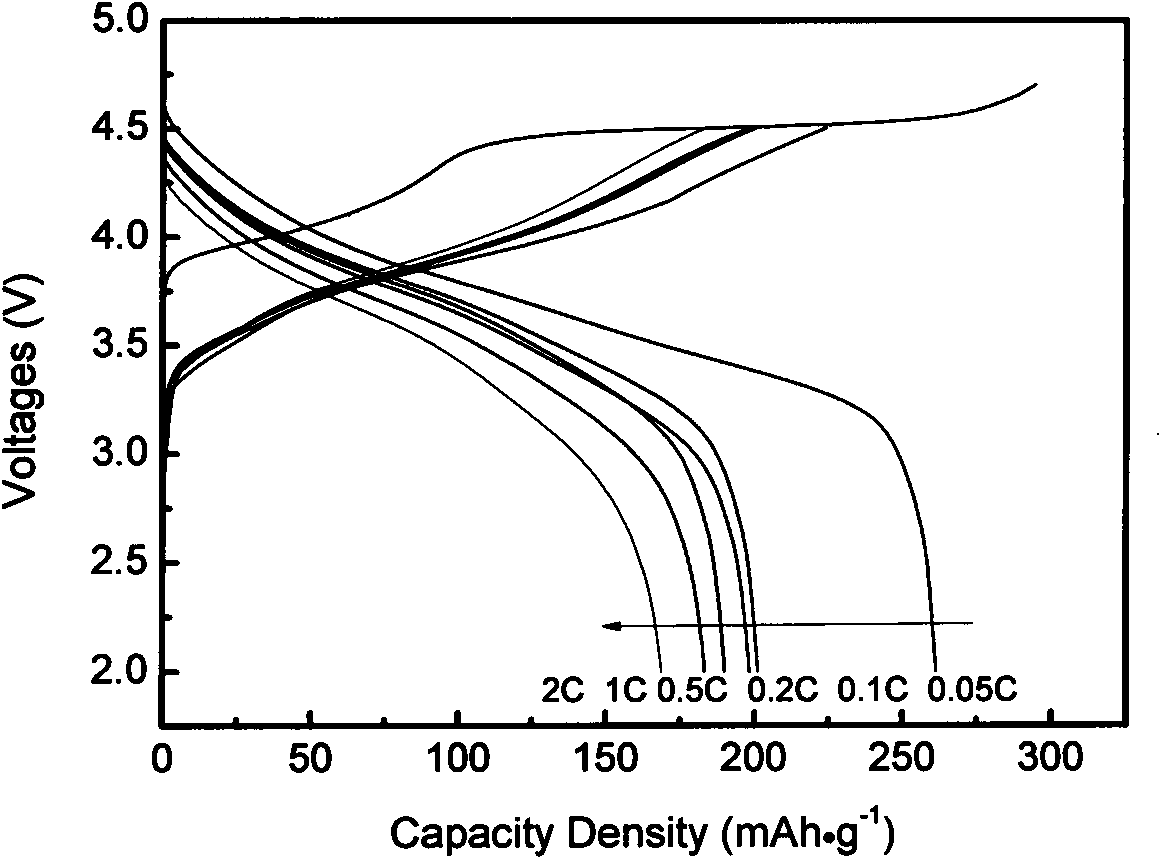
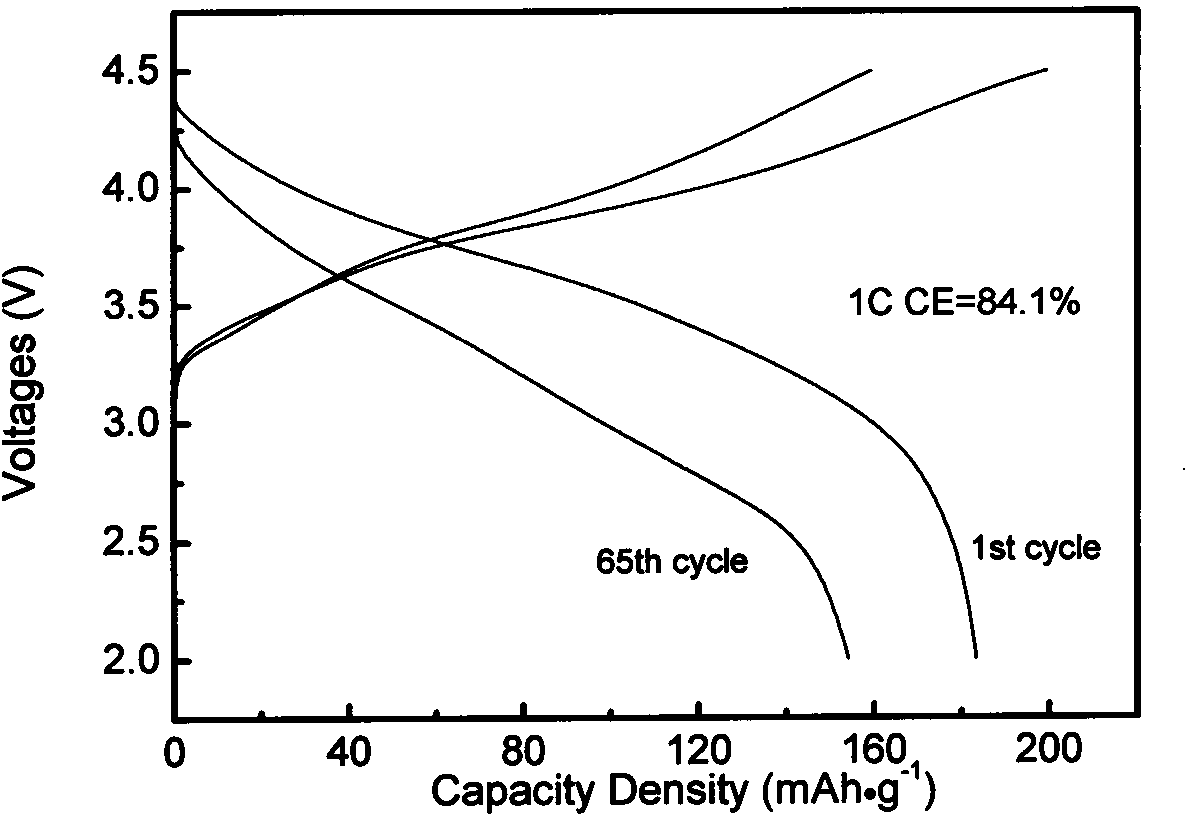
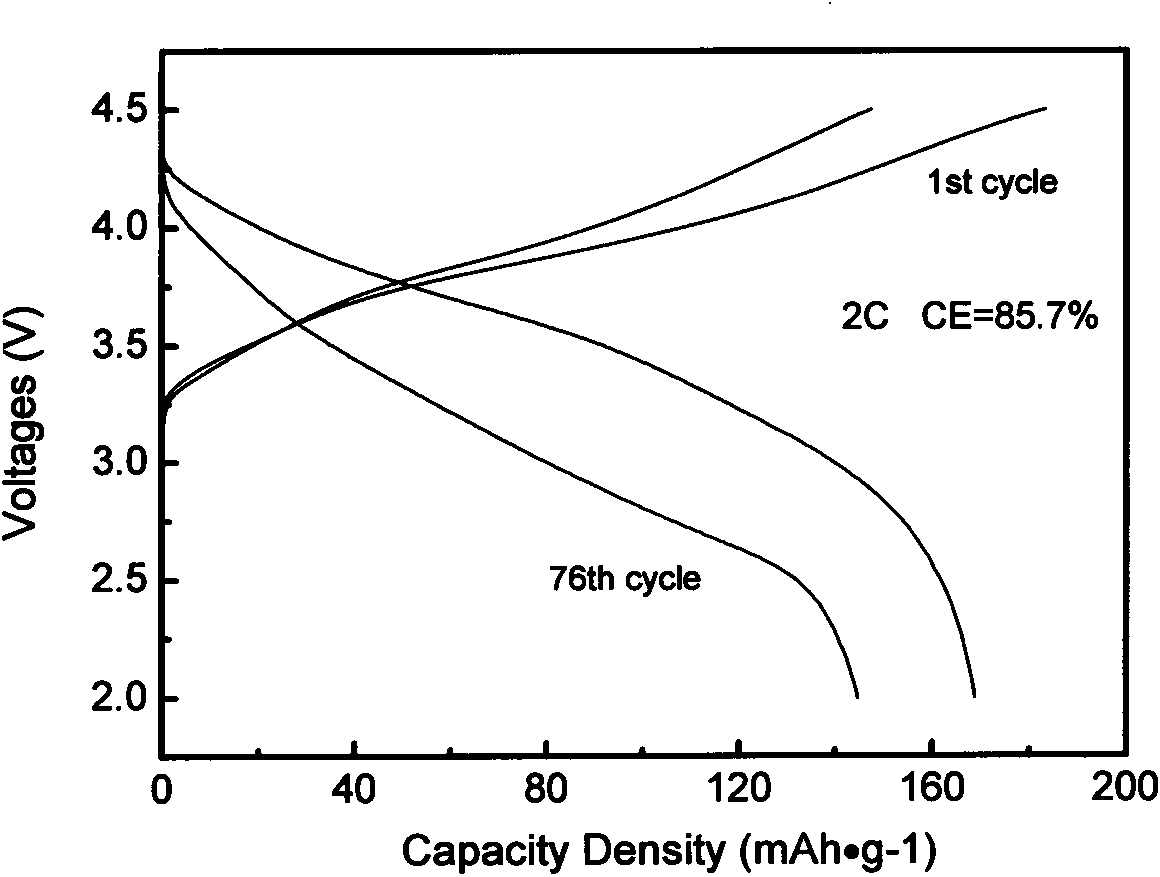
[0030] Co-precipitated lithium-rich cathode material Li 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 As coating object, La(NO 3 ) 3 ·6H 2 O, Sr(NO 3 ) 3 , manganese acetate as coating material. The powder prepared by burning the above-mentioned nitrate with glycine method was pre-fired at 800°C and mechanically mixed with lithium-rich positive electrode materials, and then sintered at 850°C to prepare Li with a coating content of 1.5wt%. 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 Cathode material. Coated Li with a mass ratio of 80:10:10 1.2 mn 0.54 co 0.13 Ni 0.13 o 2 The sample, conductive carbon black and polyvinylidene fluoride were evenly mixed to make a working electrode, and the negative electrode was a metal lithium sheet. figure 1 for (La 0.85 Sr 0.15 ) 0.9 MnO 3- δ Coated Li 1.2 mn 0.54 co 0.13 Ni 0.13 0 2 The subsequent charge and discharge curv
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap