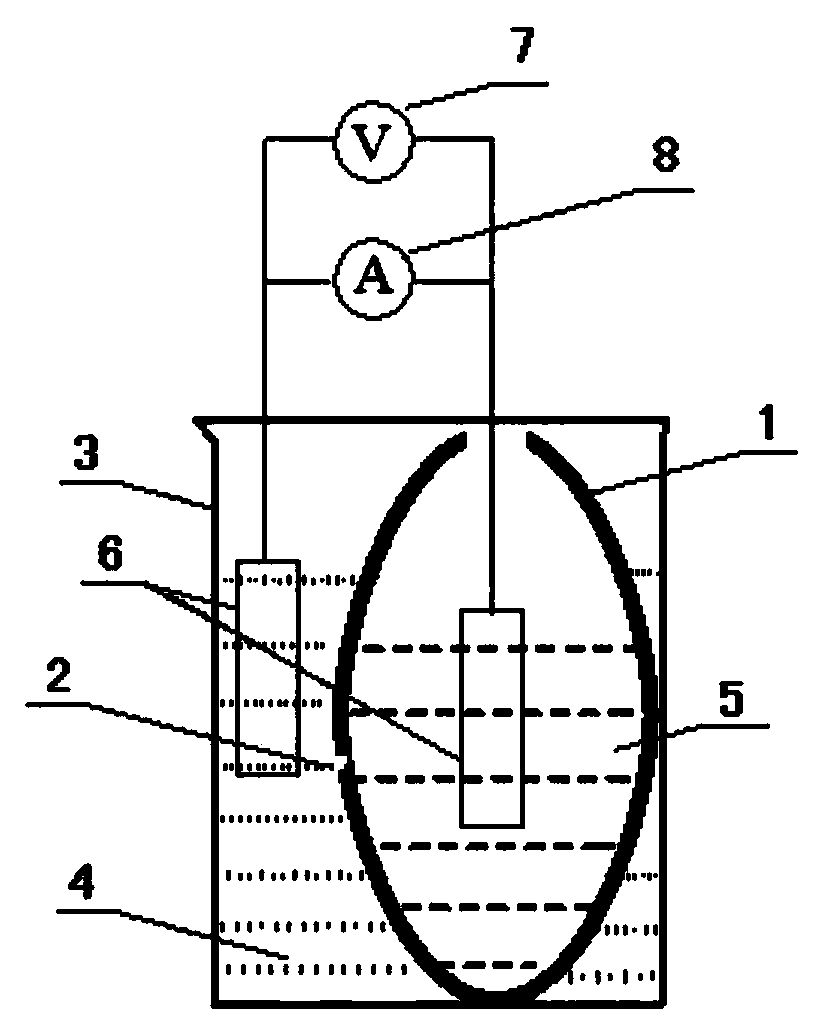
Device for oxidizing I<-> type primary battery by Cu<2+> and manufacturing method of device
A manufacturing method and primary battery technology, which can be used in educational appliances, instruments, teaching models, etc., can solve problems such as inability to achieve, and achieve the effects of short time-consuming, easy-to-obtain materials, and high reliability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
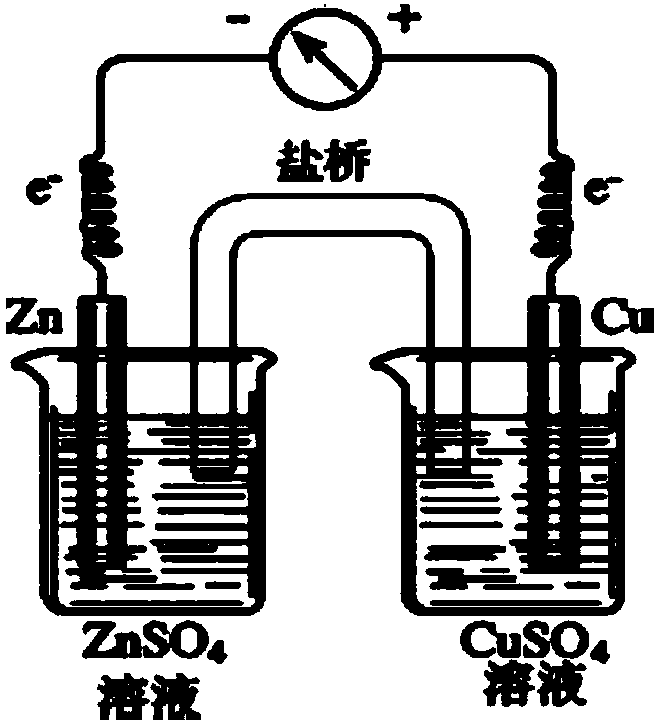
[0040] see figure 2 According to the conventional experimental device, a primary battery of 0.5mol / L zinc sulfate solution and 0.5mol / L copper sulfate solution was assembled with a salt bridge, and the deflection of the voltmeter was observed, and it was found that the pointer of the voltmeter deflected (0.6V), indicating saturation Potassium chloride-agar gel salt bridge is good, that is, usable.
Embodiment 2
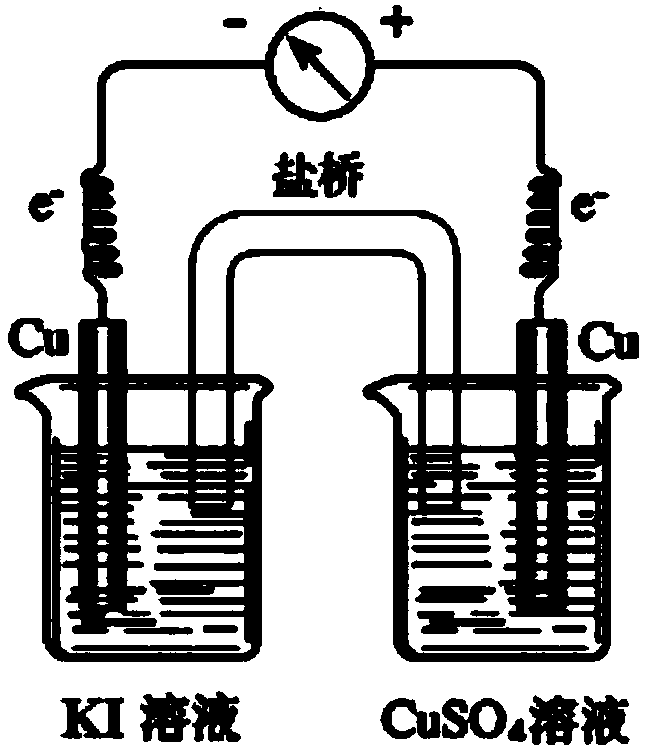
[0042] see image 3 According to the conventional experimental device, a primary battery of 0.5mol / L potassium iodide solution and 0.5mol / L copper sulfate solution was assembled with a salt bridge, and the deflection of the voltmeter was observed. It was found that the pointer of the voltmeter did not deflect, indicating that there was no electromotive force between the two electrodes. . Apparently, the device was unsuccessful.
Embodiment 3
[0044] see Figure 4 , according to the conventional experimental setup, the figure 2 and image 3 In series, that is, the primary battery assembled into 0.5mol / L zinc sulfate solution and 0.5mol / L copper sulfate solution with salt bridge is connected in series with the primary battery assembled into 0.5mol / L potassium iodide solution and 0.5mol / L copper sulfate solution with salt bridge , observe the deflection situation of voltmeter, find that voltmeter pointer deflects, and the deflection number is identical (0.6V) with embodiment 1, shows that there is no electromotive force between the potassium iodide solution of conventional experiment assembly and the primary battery electrode of copper sulfate solution, and solution ion only has The role of transfer charge further indicates that the galvanic devices assembled by conventional experiments with potassium iodide solution and copper sulfate solution were unsuccessful.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap