Preparation method of dye
A dye, dihydrogen technology, used in anthracene dyes, organic dyes, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
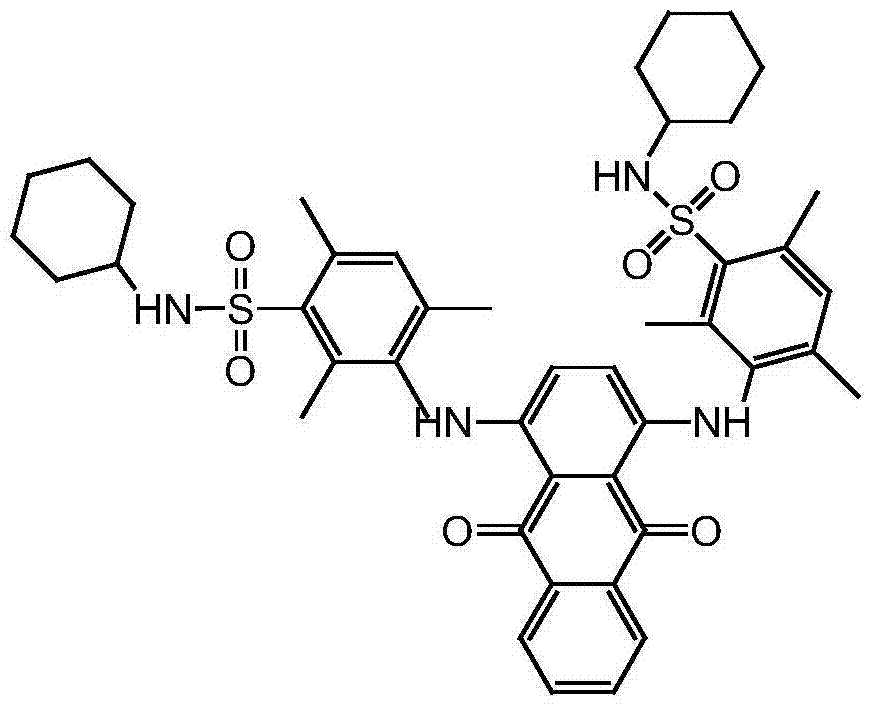
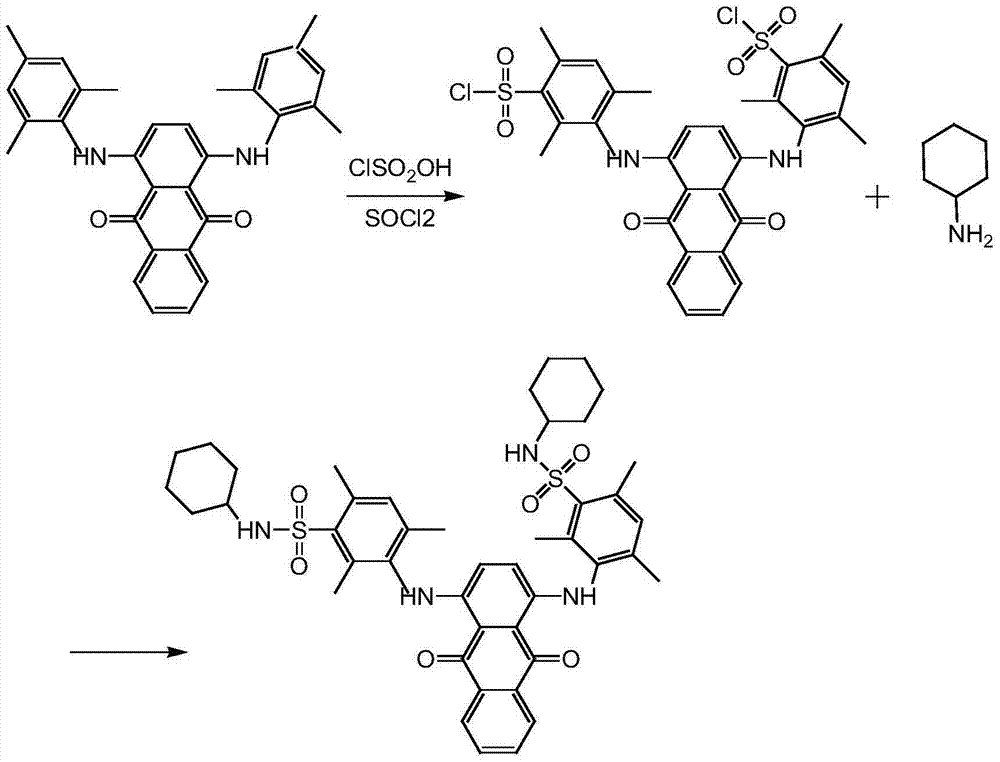
[0022] Step 13, 3'-[(9,10-dihydro-9,10-dioxo-1,4-anthracendiyl)diimino]bis(2,4,6-trimethylbenzene)sulfonyl chloride Synthesis
[0023] In a 150ml four-necked bottle with mechanical stirring and a thermometer, add 7g of solvent blue 104 and 49g of chlorosulfonic acid, stir and heat to 30°C, and keep warm for 15 minutes. After completion, the temperature was lowered to 15° C., and the reaction was kept for 7 hours. After the reaction, the reaction solution was slowly added to the ice-water mixture, the temperature was controlled at -5°C, and the addition was completed, stirred at -5°C for 30 minutes, filtered, and the filter cake was washed with cold water to obtain 22 g of the wet product of the sulfonyl chloride compound. Content 50.5%, purity 80.2%;
Embodiment 2
[0025] Step 13, 3'-[(9,10-dihydro-9,10-dioxo-1,4-anthracendiyl)diimino]bis(2,4,6-trimethylbenzene)sulfonyl chloride Synthesis
[0026] Add 7g of solvent blue 104 and 84g of chlorosulfonic acid into a 150ml four-necked bottle with mechanical stirring and a thermometer, stir and heat to 40°C, keep warm for 15 minutes, start adding 6g of thionyl chloride dropwise after the warming is over, and the dropwise addition is complete Afterwards, the temperature was lowered to 23° C., and the reaction was maintained for 6 hours. After the reaction, the reaction solution was slowly added to the ice-water mixture, and the temperature was controlled at about 0°C. After the addition was completed, it was stirred at 0°C for 30 minutes, filtered, and the filter cake was washed with cold water to obtain 23.5 g of the wet product of the sulfonyl chloride compound. Content 51.5%, purity 83.1%;
Embodiment 3
[0028] Step 13, 3'-[(9,10-dihydro-9,10-dioxo-1,4-anthracendiyl)diimino]bis(2,4,6-trimethylbenzene)sulfonyl chloride Synthesis
[0029] Add 7g of solvent blue 104 and 84g of chlorosulfonic acid to a 150ml four-necked bottle with mechanical stirring and a thermometer, stir and heat to 35°C, keep warm for 15 minutes, start adding 4g of thionyl chloride dropwise after the warming is over, and the dropwise addition is complete Afterwards, the temperature was lowered to 15° C., and the reaction was maintained for 8 hours. After the reaction, the reaction solution was slowly added to the ice-water mixture, the temperature was not higher than 15°C, after the addition was completed, it was stirred at 15°C for 30 minutes, filtered, and the filter cake was washed with cold water to obtain 21.5g of the wet product of the sulfonyl chloride compound. Content 52.1%, purity 80.1%;
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap