UV photocuring product
A light-curing, product technology, used in organic chemistry, coatings, fire-retardant coatings, etc., can solve the problems of large moisture influence, limited curing depth, slow polymerization speed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
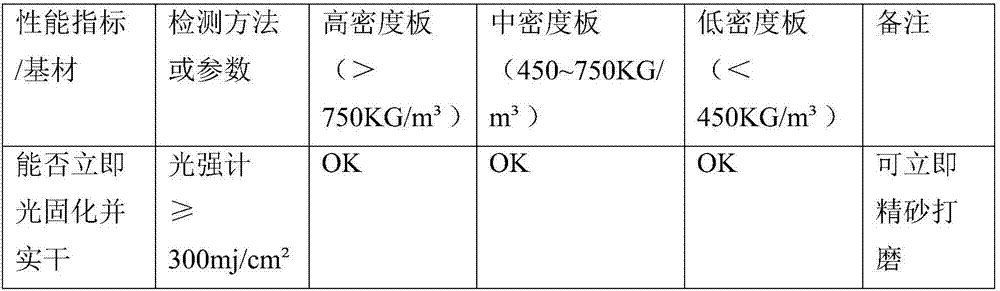
[0023] Selection of cationic photoinitiators and free radical photoinitiators, substrate wetting agents, defoamers, leveling agents, and hydroxyl-containing acrylate reactive monomers:
[0024] Cationic photoinitiator UVI 6976 (Dow Chemical, triarylsulfonium salt),
[0025] Free radical photoinitiator Irgacure 907 (Ciba, α-aminoketone derivative) / BMS (double bond, 4-p-tolylmercaptobenzophenone),
[0026] Substrate wetting agent HANSA1065 (BEZEMA), defoamer TEGO 920 (EVONIC), leveling agent EFKA3600 (BASF), hydroxypropyl methacrylate, pentaerythritol triacrylate.
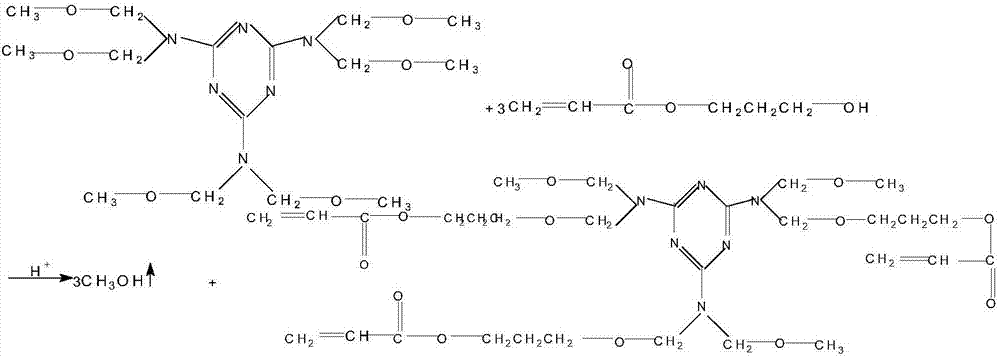
[0027] Synthesis of acrylate photosensitive prepolymers containing aminoalkoxy groups:
[0028] Add hydroxypropyl acrylate and hexamethoxymethyl melamine to the reactor, the molar ratio is 3.05:1, add polymerization inhibitor p-hydroxyanisole and catalyst p-toluenesulfonic acid, the consumption is 0.1% of the total weight of reactants , reacted at 105°C, turned on the cooling water of the horizontal condenser, and b
Embodiment 2
[0037] Embodiment 2: A kind of UV light curing product that can realize ultra-fast UV curing under normal temperature:
[0038] Cationic photoinitiator and free radical photoinitiator, UV special color paste, matting agent, filler, substrate wetting agent, wetting and dispersing agent, defoamer, leveling agent, paint tank internal stabilizer, hydroxyl-containing acrylic acid Selection of ester reactive monomers:
[0039] Cationic photoinitiator UVI 6992 (Dow Chemical, triarylsulfonium salt),
[0040] Free radical photoinitiator Irgacure 819 (Ciba, acyl phosphine oxide) / 369 (Jiu Ri, α-amino ketone derivative), UV special color paste (B7 carbon black, W28 titanium white, R254 positive red, Y139 orange, Y154 Golden, B15:3 Phthalocyanine Blue, Iron Oxide Yellow, Iron Oxide Red, G7 Phthalocyanine Green, V23 Permanent Violet, O73 Fresh Orange, etc.),
[0041] Matting agent GRACE Rad 2105, substrate wetting agent HANSA1065 (BEZEMA), wetting and dispersing agent BYK168, defoamer TEGO
Embodiment 3
[0051] Selection of cationic photoinitiators and free radical photoinitiators, pigments and fillers, substrate wetting agents, wetting and dispersing agents, defoamers, leveling agents, and hydroxyl-containing acrylate active monomers:
[0052] Cationic photoinitiator Irgacure 261 (CIBA, ferrocene salt),
[0053] Free radical photoinitiator Irgacure 907 (Ciba, α-amino ketone derivative) / ITX (double bond, thioxanthone),
[0054] Pigments (titanium dioxide, carbon black, positive red, orange, golden, phthalocyanine blue, phthalocyanine green, bright orange, permanent violet, etc.),
[0055] Filler (calcium carbonate, talcum powder, barium white, fumed silicon, etc.), substrate wetting agent HANSA1065 (BEZEMA), wetting and dispersing agent lubrizol36000 ((LUBRIZOL) \ defoamer TEGO 920 (EVONIC), leveling agent EFKA3600 ( BASF), hydroxyethyl methacrylate, dipentaerythritol pentaacrylate.
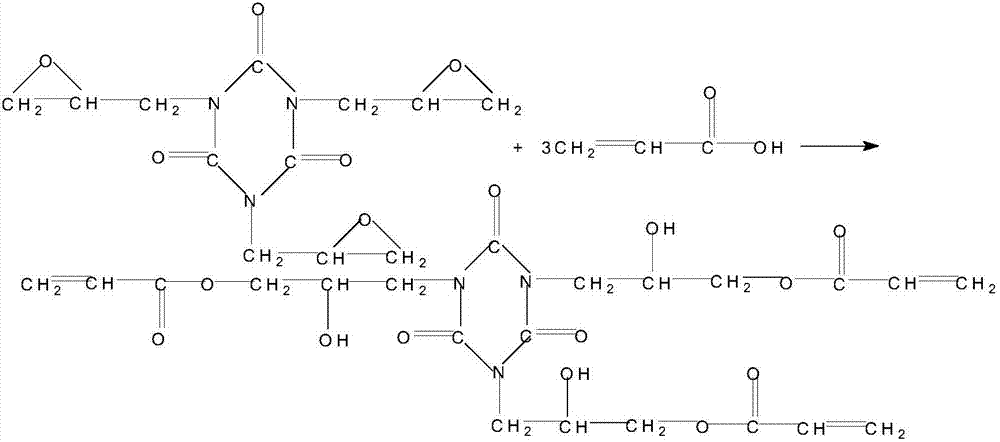
[0056] Synthesis of acrylate photosensitive prepolymers containing aminoalkoxy groups:
[0
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap