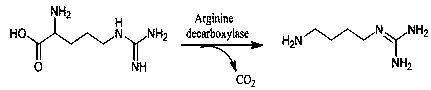
Method using thallus whole cells to catalyze arginine to convert into agmatine
A technology of agmatine and whole cells, which is applied in the field of bioengineering, can solve the problems of cell crushing, purification and extraction, and increase the burden, and achieve the effects of high conversion efficiency, easy crystallization and purification, and short fermentation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0037] Example 1: Optimization of the crystallization conditions of agmatine
[0038] Since the existing scientific research data has not yet found relevant information on the crystallization of agmatine, the present invention investigates the effects of various organic solvents on the crystallization of agmatine, including methanol, ethanol and the like. Although these organic solvents are beneficial to the crystallization of agmatine to a certain extent, considering the safety factors of agmatine in application, the present invention focuses on the influence of ethanol on the crystallization of agmatine. Because low temperature is conducive to the precipitation of agmatine, the temperature during the experiment was controlled at 4~15℃, the concentration of agmatine in the converted clear liquid was 200~300g / L, and it was concentrated in vacuum to 1 / of the original volume. 2. The final result shows that adding 55% ethanol to the concentrate is more conducive to the recovery of
Example Embodiment
[0040] Example 2: Method for catalyzing the conversion of arginine to agmatine using whole cells of bacteria
[0041] The method includes the following steps:
[0042] (1) Preparation of whole cell arginine decarboxylase concentrate
[0043] a. Construction of arginine decarboxylase genetically engineered bacteria
[0044] The genetically engineered bacteria uses Escherichia coli K12 as a host, pET28A as a vector, and adiA as a target gene. The nucleotide sequence of the adiA gene is shown in SEQ ID NO. 1, and can be constructed using conventional molecular cloning techniques.
[0045] b. Pilot fermentation culture of genetically engineered arginine decarboxylase bacteria
[0046] s 1 Take the engineered bacteria glycerin tube to inoculate the activated bacteria on the petri dish for 24 hours; store the petri dish in a refrigerator at 4°C; use an inoculation loop to dig out a loop of plate seeds under aseptic conditions and inoculate the seed culture Base (50mL / 500mL Erlenmeyer flask).
Example Embodiment
[0059] Example 3: Method for catalyzing the conversion of arginine to agmatine using whole cells of bacterial cells
[0060] The method includes the following steps:
[0061] (1) Preparation of whole cell arginine decarboxylase concentrate
[0062] a. Construction of arginine decarboxylase genetically engineered bacteria
[0063] The genetically engineered bacteria uses Escherichia coli K12 as a host, pET28A as a vector, and adiA as a target gene. The nucleotide sequence of the adiA gene is shown in SEQ ID NO. 1, and can be constructed using conventional molecular cloning techniques.
[0064] b. Pilot fermentation culture of genetically engineered arginine decarboxylase bacteria
[0065] s 1 Take the engineered bacteria glycerin tube to inoculate the activated bacteria on the petri dish for 24 hours; store the petri dish in a refrigerator at 4°C; use an inoculation loop to dig out a loop of plate seeds under aseptic conditions and inoculate the seed culture Base (50mL / 500mL Erlenmeyer fl
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap