Swine composite interferon used for emergent prevention for African swine fever
An African swine fever and compound technology, applied in the field of porcine compound interferon, can solve the problems of inability to replicate the virus and inability to combine viral nucleic acids, and achieve the effects of good immune protection, long storage period, and significant application and promotion value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
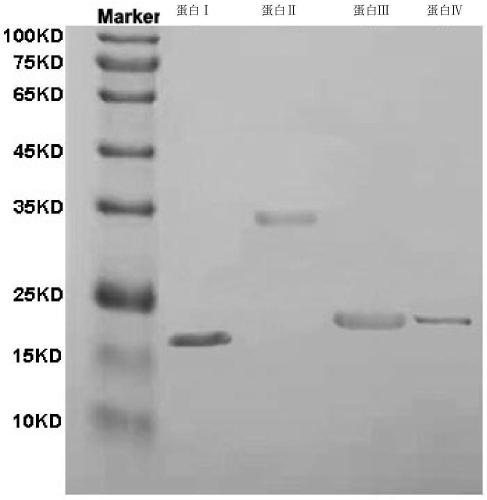
[0034] Embodiment 1, the preparation of protein solution
[0035] 1. Preparation of recombinant plasmids
[0036] The double-stranded DNA molecule shown in nucleotides 4-435 of Sequence 2 in the sequence listing was inserted between the NdeI and XhoI restriction sites of the pET30a(+) vector to obtain recombinant plasmid I. The recombinant plasmid I has an open reading frame shown in sequence 2 of the sequence listing, and expresses the protein shown in sequence 1 of the sequence listing.
[0037] The double-stranded DNA molecule shown in nucleotides 4-948 of Sequence 4 in the sequence listing was inserted between the NdeI and XhoI restriction sites of the pET30a(+) vector to obtain recombinant plasmid II. The recombinant plasmid II has an open reading frame shown in sequence 4 of the sequence listing, and expresses the protein shown in sequence 3 of the sequence listing.
[0038] Insert the double-stranded DNA molecule shown in nucleotides 4-552 of Sequence 6 in the sequence l
Embodiment 2
[0080] Embodiment 2, detect interferon activity
[0081] The test solutions were: the protein I solution, protein II solution, protein III solution or protein IV solution prepared in Example 1, all of which were freshly prepared.
[0082] 1. Cell plating
[0083] Get well-grown PK-15 cells, digest with trypsin, and then suspend with DMEM medium containing 100 U / mL penicillin, 100 U / mL streptomycin and 10% (volume ratio) FBS to obtain a cell concentration of 1 × 10 6 cell suspension per mL, add the cell suspension to a 96-well cell culture plate (100 μl / well), and then place it at 37°C with 5% CO 2 Cultured in a static incubator for 8 hours (to become a monolayer of cells).
[0084] 2. Take the test solution and dilute to 25 times volume, 250 times volume, 2500 times volume or 25000 times volume with DMEM medium containing 10% (volume ratio) FBS to obtain each dilution.
[0085] 3. Take the cell culture plate that completed step 1, suck and discard the supernatant; add th
Embodiment 3
[0094] Embodiment 3, the stability of protein solution
[0095] The freshly prepared protein I solution, protein II solution, protein III solution or protein IV solution prepared in Example 1 was placed at room temperature for 3 months, and then used as the test solution (10 parallel treatments were set for each solution).
[0096] 1. Morphological observation
[0097] The protein Ⅰ solution, protein Ⅱ solution and protein Ⅲ solution all became turbid after standing at room temperature for 3 months.
[0098] The protein Ⅳ solution remained clear after 3 months at room temperature.
[0099] 2. Detection of interferon activity
[0100] Method is with embodiment 2. The results are averaged.
[0101] The interferon titer of the protein Ⅰ solution after 3 months at room temperature was 8.2×10 5 U / ml, 48% of freshly prepared.
[0102] The interferon titer of the protein Ⅱ solution after 3 months at room temperature was 3.3×10 5 U / ml, 43% of freshly prepared.
[0103] The in
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap