Bridged tetraphenylethylene-based supramolecular polymer light capture system, and preparation and application thereof
A technology of supramolecular polymer and tetraphenylethylene, which is applied in the direction of luminescent materials, chemical instruments and methods, can solve the problems of cumbersome synthesis steps, restricted development, and large environmental pollution, and achieve perfect spectral characteristics, stable structure, and low production cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Compound M:
[0052] In a 250mL three-necked flask, add compound P (2.58g, 5.5mmol), add compound Q (3.60g, 11.9mmol), add dry chloroform (CHCl 3 , 60mL), stirred at room temperature for 12h. Post-treatment: add 20 mL of chloroform, wash with 1M hydrochloric acid (80 mL), and saturated NaHCO 3 solution, saturated NaCl solution, and anhydrous MgSO 4 Dry, filter with suction, spin off the solvent to about 5mL, add dropwise to MeOH stirred at high speed, a white solid precipitates, reflux for 3 hours, and filter with suction to obtain a white solid powder which is compound M (4.27g, 4.51mmol), the yield was 82%.
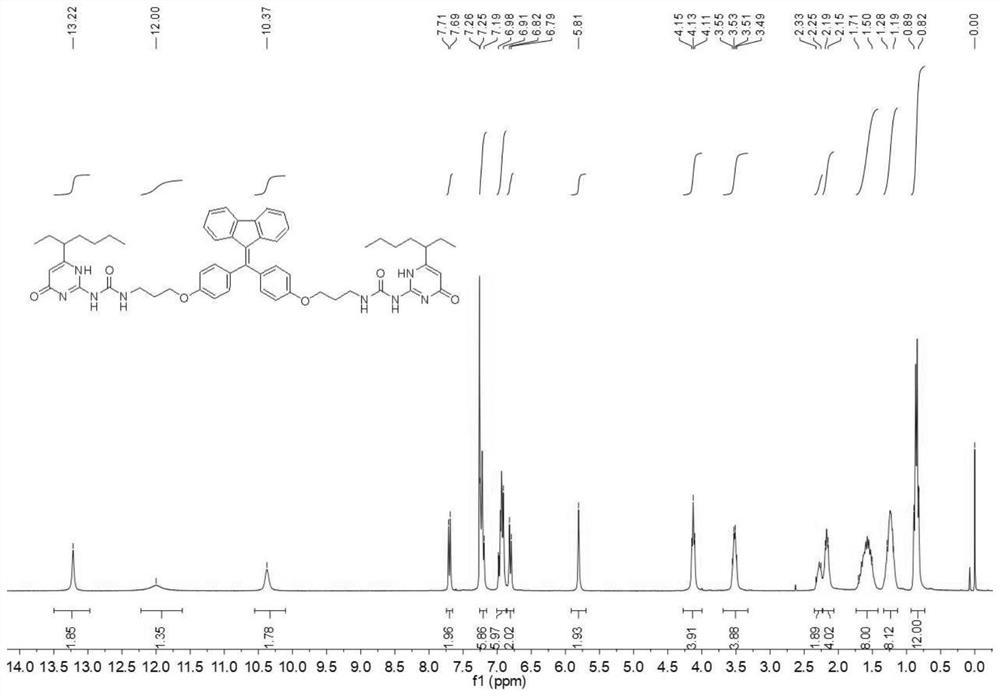
[0053] The H NMR spectrum of compound M is as image 3 shown. 1 H NMR (300MHz, CDCl 3 ):δ(ppm)=13.22(s,2H,NH),12.00(s,2H),10.37(s,2H),7.70(d,J=7.6Hz,2H),7.25-7.19(m,6H) ,6.99-6.90(m,6H),6.81(d,J=7.8Hz,2H),5.81(s,2H),4.13(t,J=6.3Hz,4H),3.56-3.48(m,4H), 2.33-2.25 (m, 2H), 2.20-2.15 (m, 4H), 1.71-1.50 (m, 8H), 1.29-1.19 (m, 8H), 0.90-0.82 (m, 12H
Embodiment 2
[0057] Preparation of Supramolecular Polymer Light Harvesting System:
[0058] Step 1, weigh 91mg cetyl ammonium bromide to a 250mL volumetric flask, dilute to 250mL with deionized water, and prepare an aqueous solution with a concentration of 1.0mmol / L;
[0059] Step 2, weigh 96.1mg of compound M into a 10mL volumetric flask, add chloroform to make up to 10mL, and prepare a 10mmol / L mother solution.
[0060] Step 3, weigh 5.2mg of compound NDI into a 10mL volumetric flask, add chloroform to make up to 10mL, and prepare a 1.0mmol / L mother liquor;
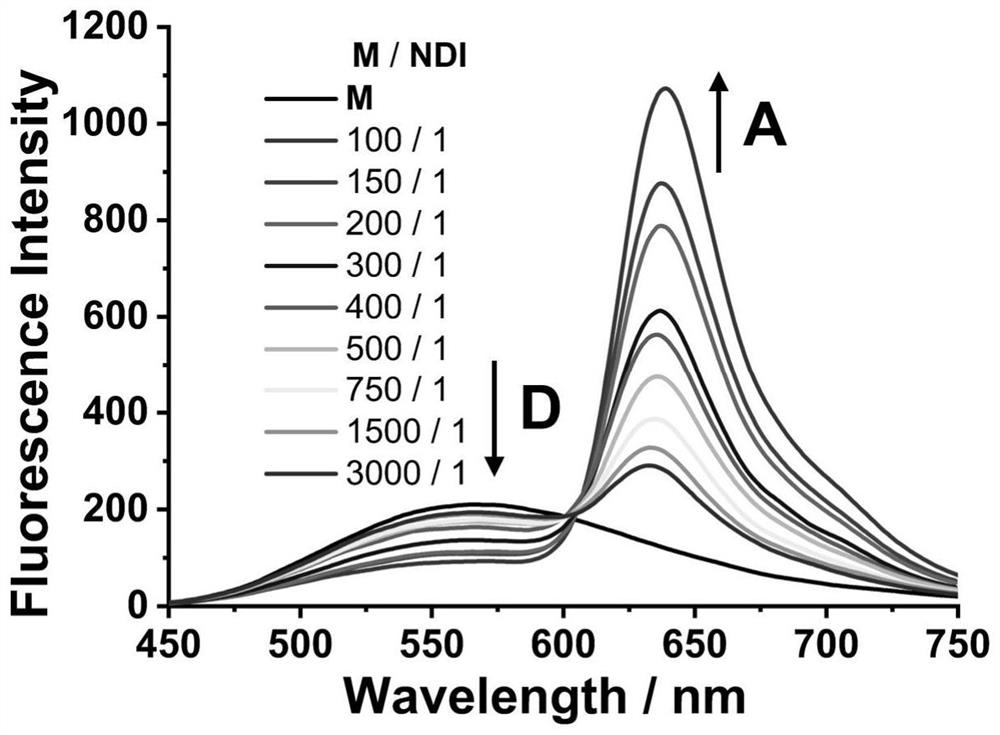
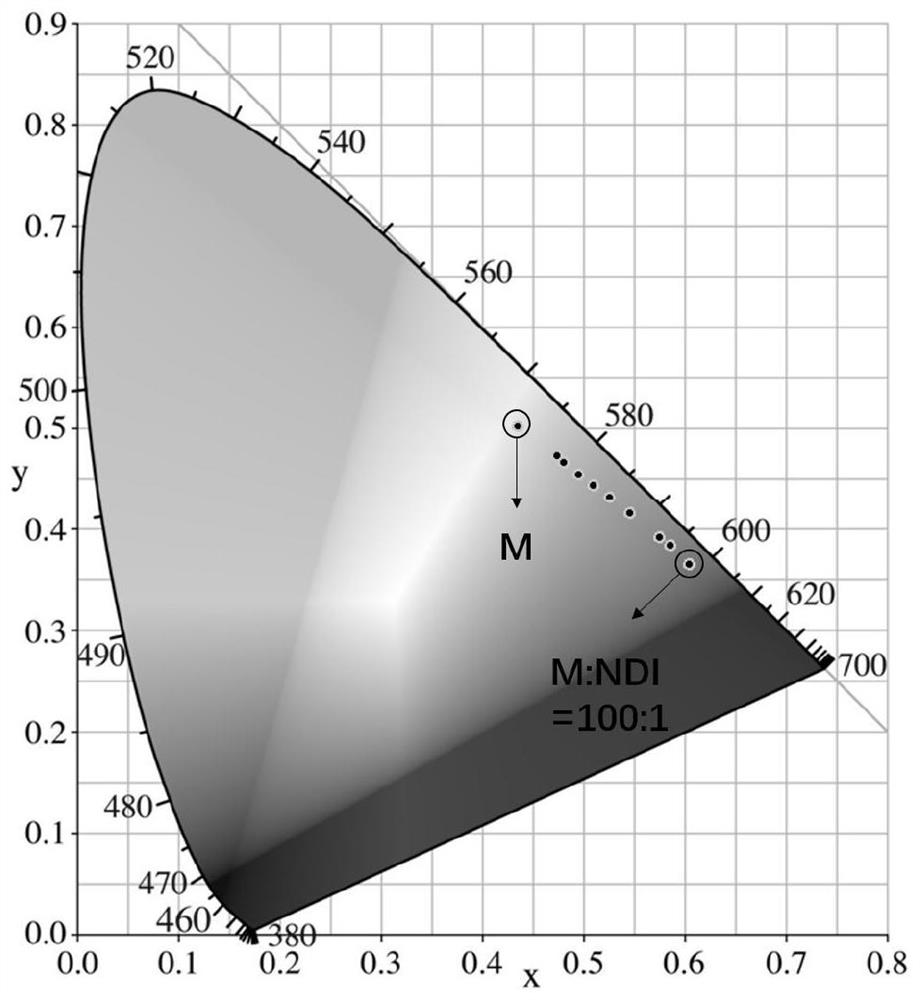
[0061] Step 4, pipette the mother liquor of compound M and the mother liquor of compound NDI respectively with a pipette gun, add to a 10mL volumetric flask and mix well, then add CTAB aqueous solution to constant volume, form aqueous phase dispersed nanoparticles after ultrasonication for 30min, wherein compound M The concentration is 5×10 -5 mol / L, the corresponding M / NDI concentration ratios are 100 / 1, 150 / 1, 200 / 1
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap