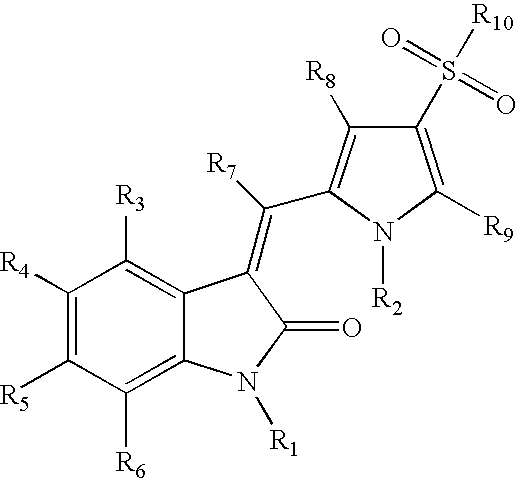
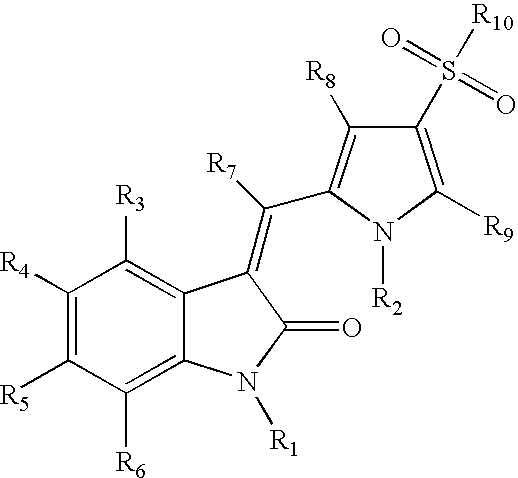
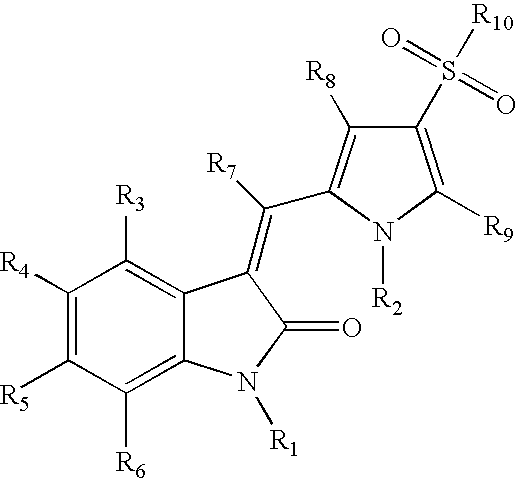
Sulfonylated pyrrole-2-indolinone derivatives as kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Example 1
3-{4-(2-Dimethylcarbamoyl-ethyl)-2-methyl-5-[2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-1H-pyrrole-3-sulfonyl}-benzoic acid
Oxindole (Aldrich) was condensed with 3-[4-(2-dimethylcarbamoyl-ethyl)-5-formyl-2-methyl-1H-pyrrole-3-sulfonyl]-benzoic acid and piperidine in ethanol to give the titled compound.
Example
Example 2
3-{4-(2-Dimethylcarbamoyl-ethyl)-5-[5-fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-2-methyl-1H-pyrrole-3-sulfonyl}-benzoic acid
5-Fluorooxindole (Combi-block) was condensed with 3-[4-(2-dimethylcarbamoyl-ethyl)-5-formyl-2-methyl-1H-pyrrole-3-sulfonyl]-benzoic acid (prepared as described above in the synthesis of aldehyde 7) and piperidine in ethanol to give the titled compound.
1HNMR (400 MHz, DMSO-d6) δ 8.25 (s, 1H), 8.06 (d, 1H), 7.81 (d, 1H), 7.72 (m, 2H), 7.50 (m, 1H), 6.96 (m, 1H), 6.84 (m, 1H), 3.10 (m, 2H), 2.76 (s, 3H, CH3), 2.70 (s, 3H, CH3), 2.58 (s, 3H, CH3), 2.29 (m, 2H).
MS m / z 524 [M−1].
Example
Example 3
3-[5-[5-Chloro-2-oxo-1,2-dihydrb-indol-(3Z)-ylidenemethyl]-4-(2-dimethylcarbamoyl-ethyl)-2-methyl-1H-pyrrole-3-sulfonyl]-benzoic acid
5-Chlorooxindole (Aldrich) was condensed with 3-[4-(2-dimethylcarbamoyl-ethyl)-5-formyl-2-methyl-1H-pyrrole-3-sulfonyl]-benzoic acid (prepared as described above in the synthesis of aldehyde 7) and piperidine in ethanol to give the titled compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Catalytic activity | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
| Disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap