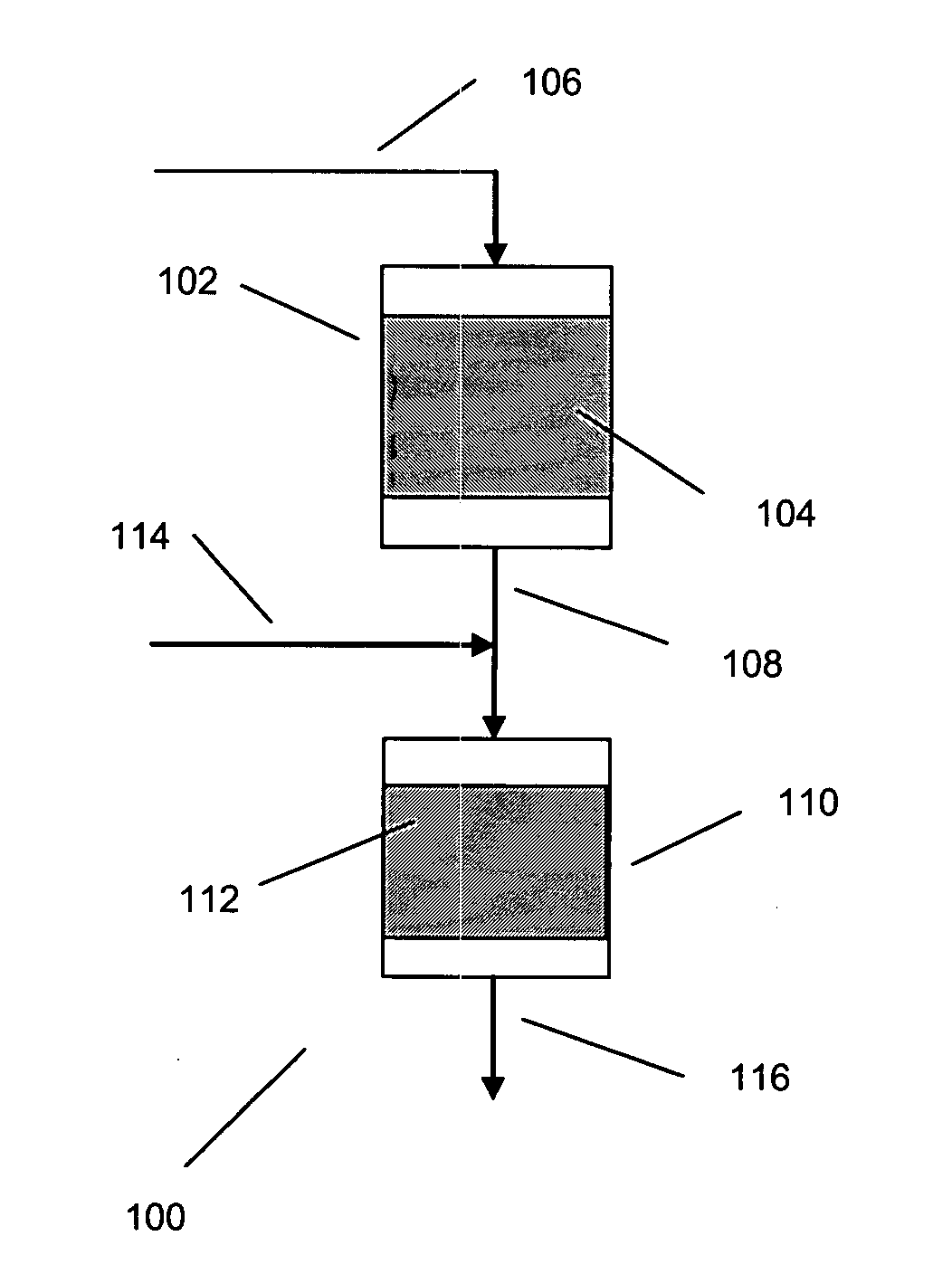
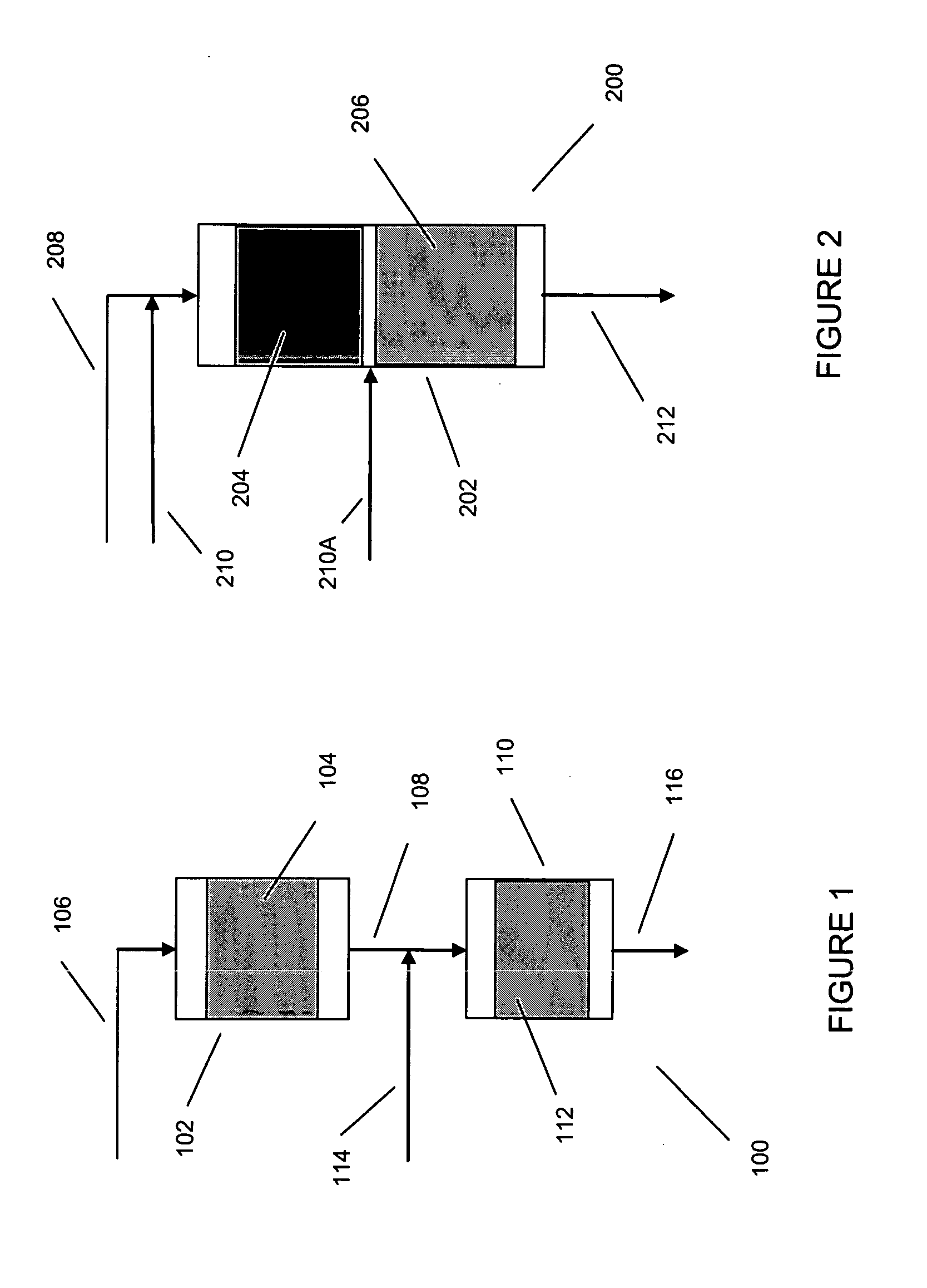
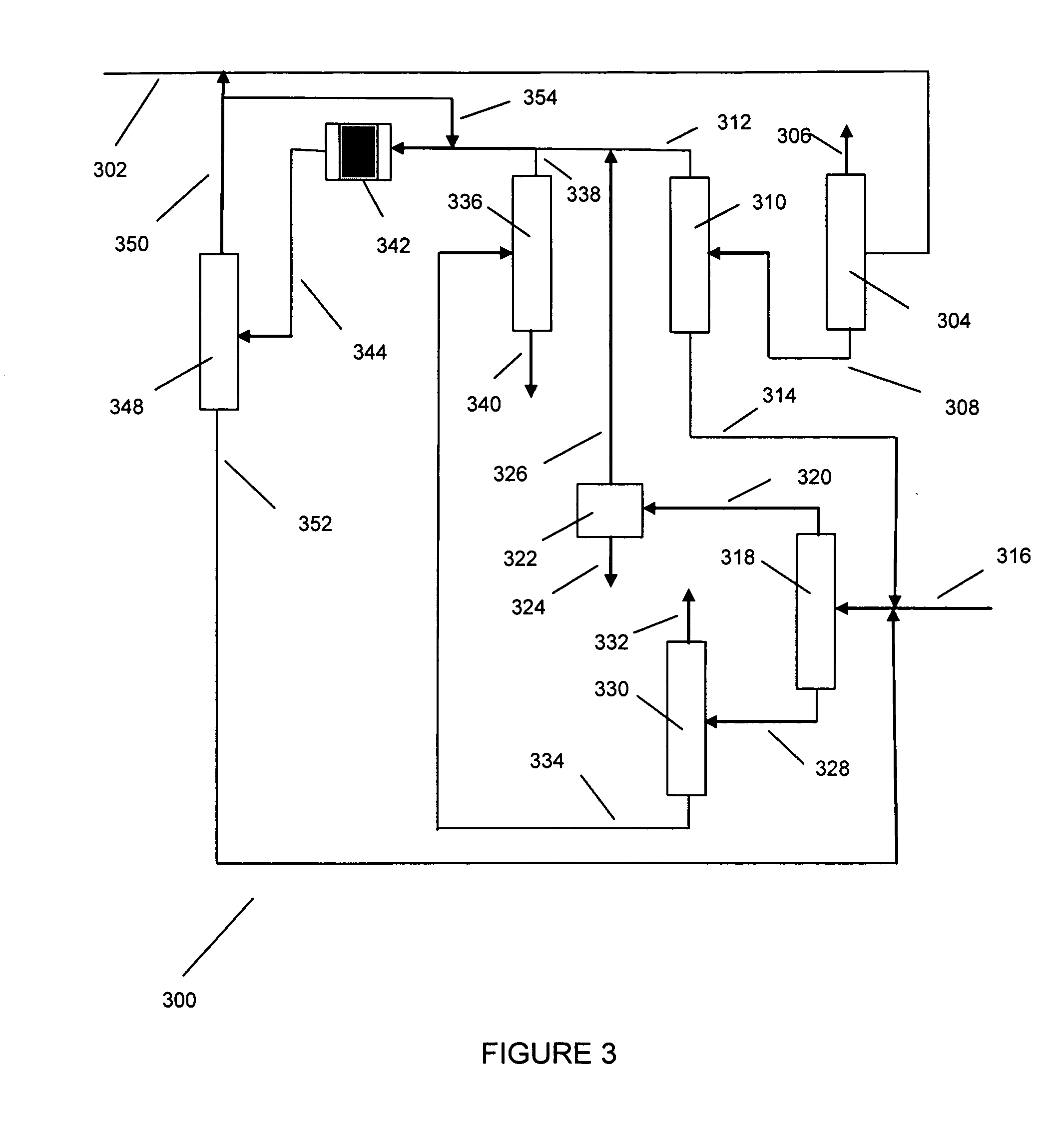
Processes for producing xylenes using isomerization and transalkylation reactions and apparatus therefor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] Processes for the production of xylene isomer are disclosed, for instance, in Robert A. Meyers, Handbook of Petroleum Refining Processes, Second Edition, McGraw-Hill, 1997, Part 2.
[0052] In general, the feed stream for aromatics isomerization is typically a C8 aromatics stream from which one or more xylenes have been removed as product. The C8 aromatics stream from which one or more xylenes are removed is typically derived from xylene containing recycle and fresh C8 aromatics feed. Usually the fresh C8 aromatics feed is obtained from processes, such as catalytic reforming and / or extraction, for the production and recovery of aromatics from other hydrocarbons. Hence, fresh alkylaromatic for use in the present invention may be found in appropriate fractions from various refinery petroleum streams, e.g., as individual components or as certain boiling-range fractions obtained by the selective fractionation and distillation of catalytically cracked or reformed hydrocarbons. Concen
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap