Pharmaceutical compositions comprising chlorophenyl piperazine derived compounds and use of the compounds in producing medicaments
a technology of chlorophenyl piperazine and composition, which is applied in the direction of drug compositions, medical preparations, nervous disorders, etc., can solve the problems of social problems, suspicious safety, and most harmful side effects, and achieve the effect of high safety and not generating side effects on lethargy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044]The present invention will now be described more specifically with reference to the following Embodiments. It is to be noted that the following descriptions of preferred Embodiments of this invention are presented herein for purpose of illustration and description only; it is not intended to be exhaustive or to be limited to the precise form disclosed.
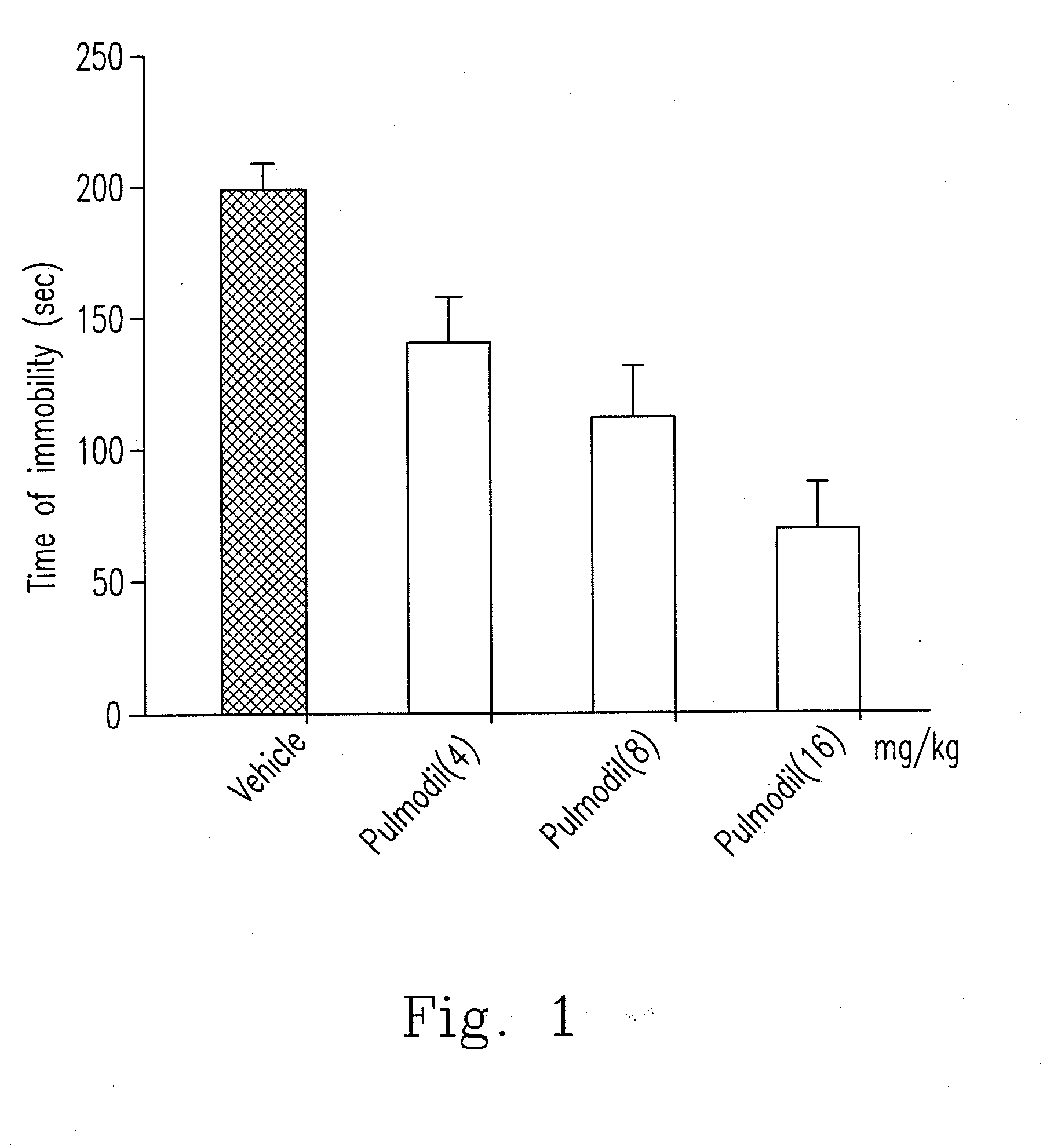
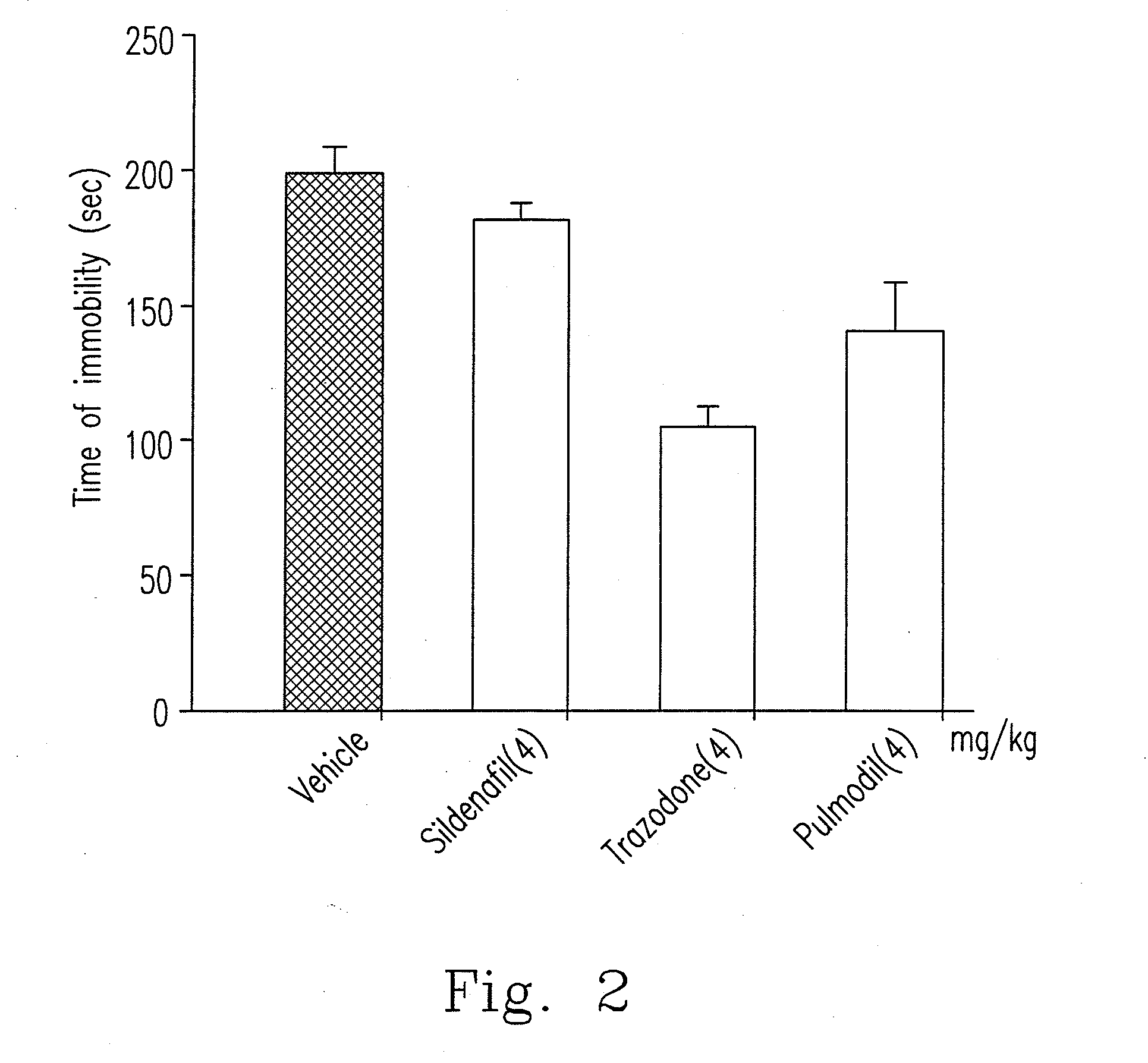
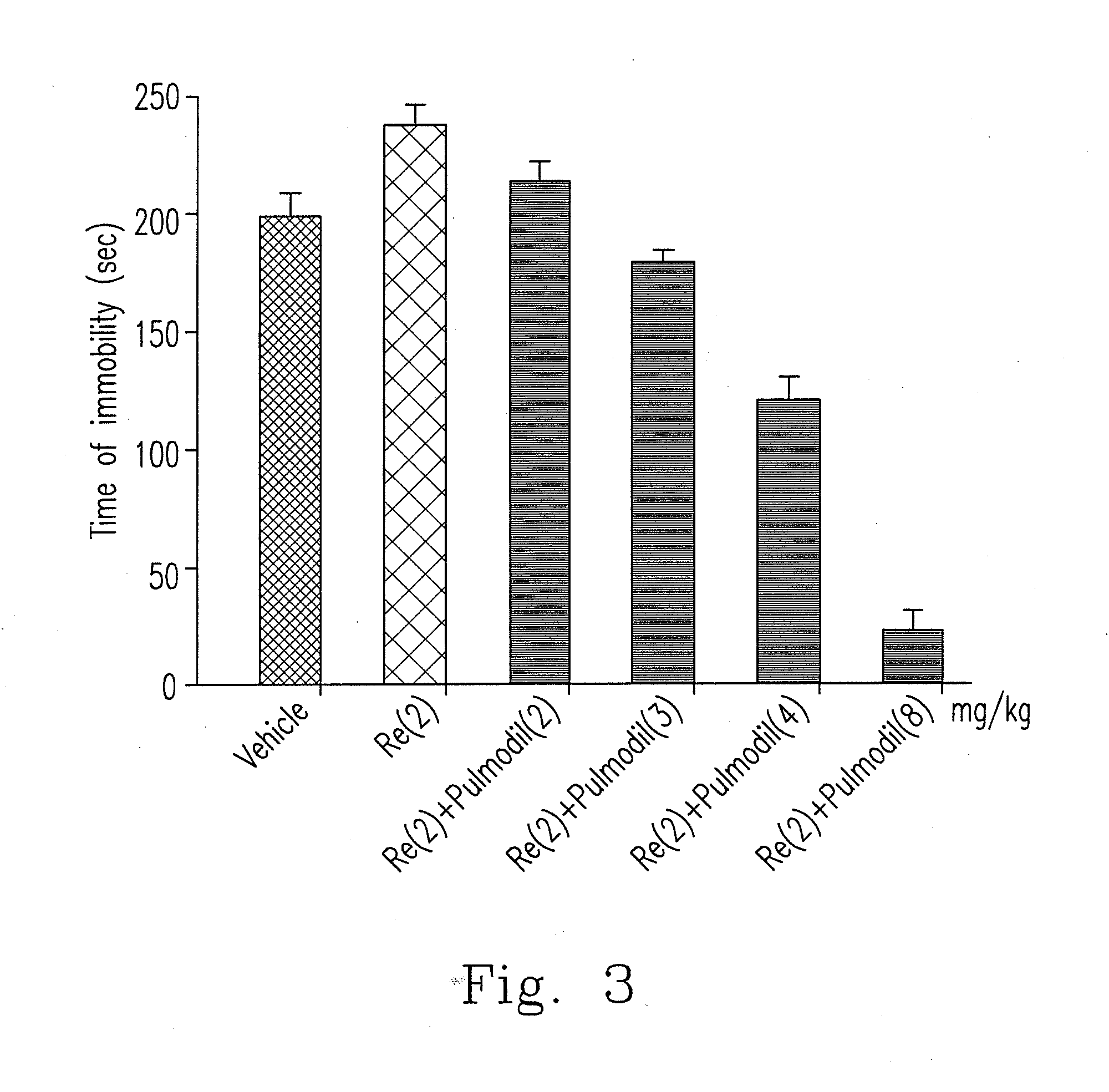
[0045]As illustrated above, xanthine-based compound is nominated as 7-[244-(2-chlorophenyl)piperazinyl]ethyl]-1,3-dimethylxanthine which has a structural formula (Formula I) as follows.
[0046]Among these, 2-chlorophenyl piperazine group is the aforementioned o-CPP, which regulates serotonergic neurotransmission and NO / cGMP pathway. In addition, xanthine-based compound does not generate lethargy effect per se since o-CPP lacks anti-histaminergic activity.
[0047]When animals are stimulated (such as psychological stress) or has abnormal physiological conditions to influence the original serotonergic neurotransmission, they might induce
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap