Bioengineered adipocytes for the light-controlled release of insulin and other peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
and Methods
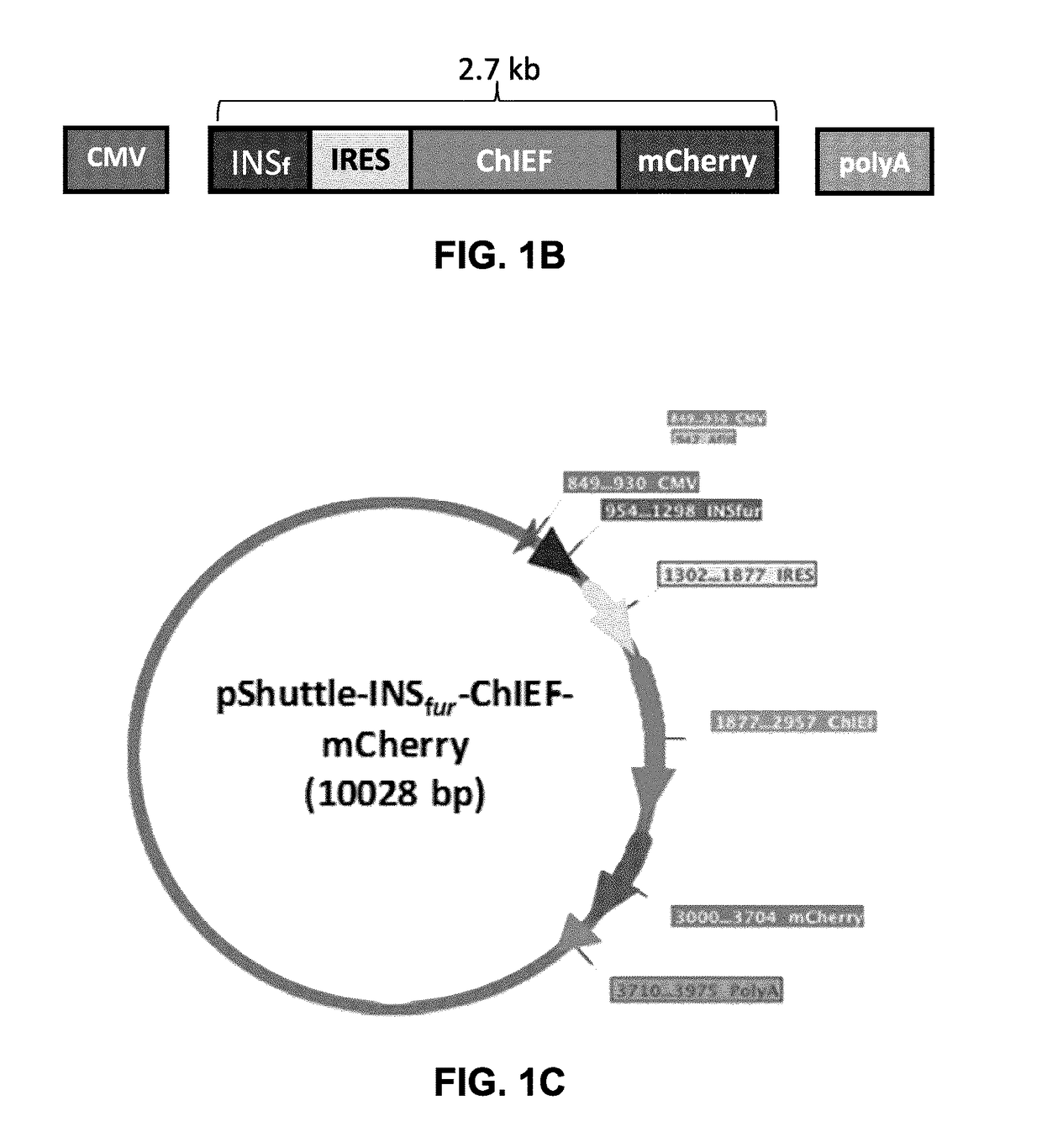
[0126]Construction of pShuttle-INSfur-ChIEF-mCherry (FIGS. 1A to D).
[0127]The INSfur cassette encodes a human leptin leader peptide followed by mutated human preproinsulin B-Chain, C-Peptide and A-Chain. Modified triplets that were introduced to encode optimal furin sites (RXKR) are indicated by F in FIG. 1A. The following DNA was synthesized by Genscript® and inserted into pUC57. The LacZ-ChiEF mCherry fragment was liberated from pUC57 with NotI and XbaI and inserted into pShuttle-CMV after cutting with the same enzymes to yield pShuttle-INSfur.
[0128]Following processing by Furin (FIG. 1D), the mature insulin secreted has only 1 mutation in its B-chain (L50R) and none in the A-chain (relative to native insulin). The leader peptide is that from human Leptin. NCBI: Leptin: NM_000230.2. The ChIEF sequence here has 2 extra N-terminal amino acids (Thr-Ser) that comprises an in-frame SpeI site (actagt). This was introduced to enable easy replacement of ChIEF with other channelrho
example 2
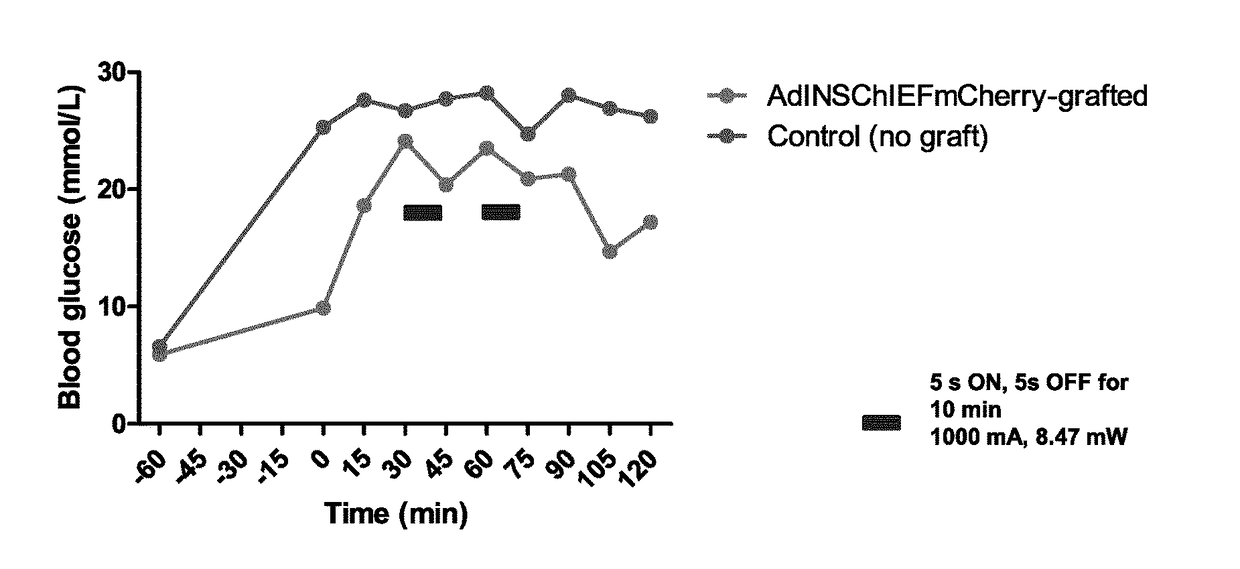
[0158]tSA201 cells (human embryonal kidney, SV40 transformed, cell line) were transfected with the ChR variant ChIEF C-terminally fused to mCherry (FIGS. 2A to 2D and 3). ChIEF has been chosen over the native ChR1 for its enhanced light-sensitivity and activation properties12. mCherry positive cells were subjected to pulses of blue light (470 nm) stimulation and they generated robust inward currents that were dependent on the duration (FIG. 2C) and intensity (FIG. 2D) of light exposure.
[0159]An adenoviral delivery vector was then constructed. The adenovirus, referred to as Ad-INS-ChIEF, encodes a leptin leader peptide followed by a modified proinsulin sequence in which the PC1 / 3 and PC2 cleavage sites of the native proinsulin have been replaced by sites optimized for cleavage by furin, a protease which is expressed in adipocytes. This bioengineering design strategy has been chosen to facilitate processing of the proinsulin peptide in adipocytes as this cell type does not express the
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bioactive | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap