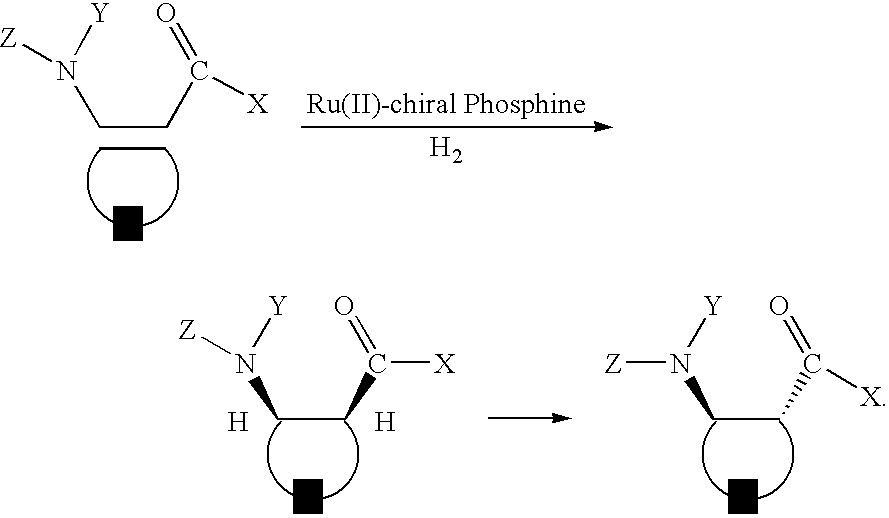
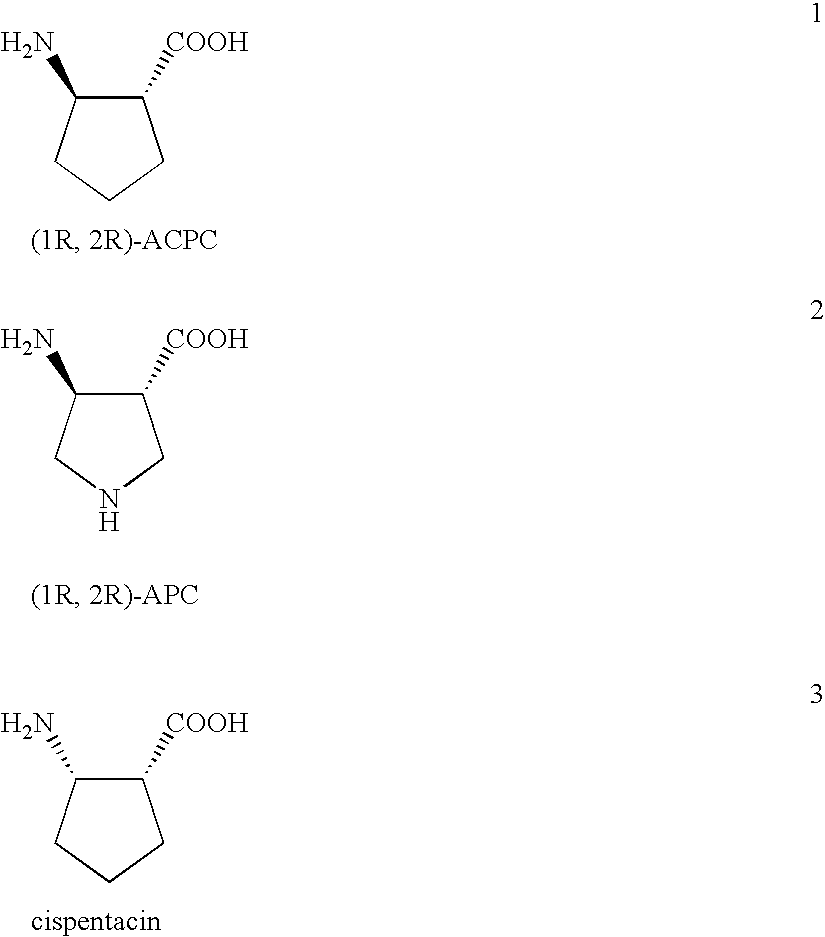
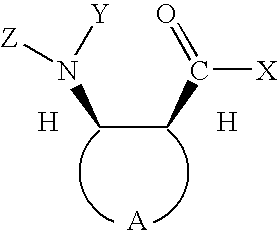
Preparation of chiral cyclic amino acids and derivatives
a technology of cyclic amino acids and derivatives, which is applied in the preparation of carbamic acid derivatives, organic chemistry, and racemisation of organic compounds, etc., can solve the problems of enantioselective hydrogenation, less success, and general difficulty in tetra-substituted olefin hydrogenation, and achieve high yield and enantioselectivity. , the effect of efficient catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
General Procedures:
[0074]All reactions and manipulations were performed in a nitrogen-filled glove box or using standard Schlenk techniques. THF and toluene were dried and distilled from sodium-benzophenone ketyl under nitrogen. Methylene chloride was distilled from CaH2. Methanol was distilled from Mg under nitrogen. (R, R)-BDNPB was made a solution of 10 mg / ml in toluene before use. Column chromatography was performed using EM silica gel 60 (230˜400 mesh). 1H, 13C and 31p NMR were recorded on Bruker WP-200, AM-300, and AMX-360 spectrometers. Chemical shifts were reported in ppm down field from tetramethylsilane with the solvent resonance as the internal standard. Optical rotation was obtained on a Perkin-Elmer 241 polarimeter. MS spectra were recorded on a KRATOS mass spectrometer MS 9 / 50 for LR-EI and HR-EI. GC analysis was carried on Hewlett-Packard 6890 gas chromatography using chiral capillary columns. HPLC analysis was carried on Waters™ 600 chromatography.
General Procedures
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap