Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4 results about "Clinical trial" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial – their approval does not mean that the therapy is 'safe' or effective, only that the trial may be conducted.
Wet packing agent for treating rheumatoid bone bi-syndrome and preparation method thereof
ActiveCN101721573AEasy to useAnthropod material medical ingredientsAntipyreticMonkshoodsClinical trial
Owner:BEIJING SINOHANFANG PHARMA SCI &TECH
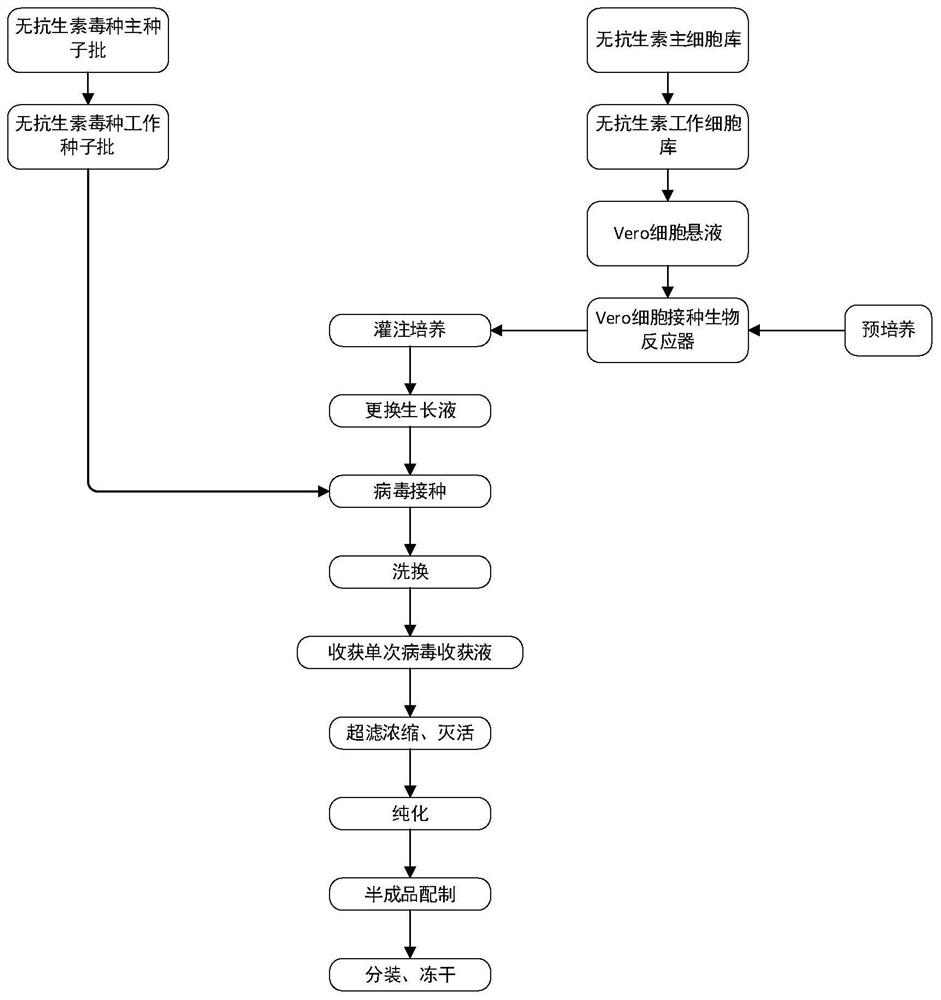
Preparation process of rabies vaccine without antibiotic addition
ActiveCN112451658AAvoid the risk of adverse reactionsReduce impuritySsRNA viruses negative-senseViral antigen ingredientsPerfusion CultureUltrafiltration
Owner:CHANGCHUN ZHUOYI BIOLOGICAL CO LTD
Health-care beverage
InactiveCN102948903ARelief and improvement of fatigue symptomsOrganic active ingredientsWine preparationCitrullinePharmacologic action
The invention belongs to the technical field of foods or medicines, and particularly relates to a health-care beverage. The health-care beverage comprises a solvent, D-ribose, citrulline and / or arginine. The health-care beverage is controllable in quality and safe in use; and proved by pharmacology and the clinical tests, the health-care beverage has good pharmacologic action and is suitable for industrial production.
Owner:郭景龙
Clinical payment network system and methods
InactiveCN102893302AEasy to handleEfficient decompositionFinanceOffice automationProgramming languageCoding block
Owner:CLINVERSE
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap