Preparation method of high-purity scutellarin crude drug
A technology of scutellarin and scutellarin, which is applied in the field of preparation of high-purity raw materials, can solve the problems of incomplete elimination, itching, rash, etc., and achieve the effects of high cost, simplified process steps, and easy selection of mechanical equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
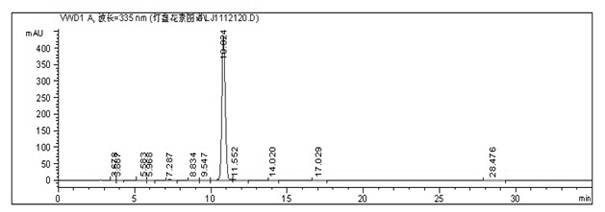
[0030] Example 1: Weigh 6.5 kg of crude raw material with 90.25% scutellarin content in the market, add 50 L of water to stir and moisten, add 20% arginine solution while stirring, heat to boiling, adjust pH value to 8.0, add water to 130L, centrifuged, clarified and filtered, and the filtrate was respectively loaded on a polyacrylate-based macroporous resin column with a diameter of 20cm and a height of 180m, and a sample load of 650g, with a sample volume of 13L, was eluted with purified water, the pressure was 4bar, and the purity of the middle section was 99% % above the yellow clarified eluate, combined the collected liquid, added glacial acetic acid to the collected liquid to adjust the pH below 3, and after natural sedimentation for 1 hour, released the supernatant, and the precipitated suspension was input into a dryer to dry to obtain scutellarin raw material drug 3.92 kg, the purity of B as determined by HPLC is 99.4014%, such as figure 1 shown.
Embodiment 2
[0031]Example 2: Weigh commercially available crude product with scutellarin content of 87.32%, 35kg of raw material, put it in from the manhole of the liquid distribution tank, open the drain valve to release 150L of water, turn on the agitator to stir and moisten, and input 30% arginine solution at the same time While stirring, turn on the steam valve to heat to boiling, control the pH value to 8.3, open the water inlet valve again to add water to a total volume of 580L and stir well, take a sample to detect the pH value is 8.25, and the content of breviscapine is 60.5mg / ml. Open the liquid discharge valve to input the liquid medicine into the high-speed tubular centrifuge, and the centrifuged liquid is input into the clarification filter, and the clarified liquid is input into a polyacrylate-based macroporous resin column with a diameter of 50 cm and a height of 400 cm. 115L, pressure 6bar, collect the yellow clarified eluate with a purity of more than 99% in the sedimentatio
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap