Preparation method of catalyst for methyl difluoroacetate hydrogenation to produce difluoroethanol
A technology of methyl difluoroacetate and difluoroethanol, which is applied in the field of catalyst preparation, can solve the problems of low catalytic reaction conversion rate, difficult continuous production control, high requirements on catalyst reaction conditions, etc., and achieves the effect of high-efficiency catalytic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
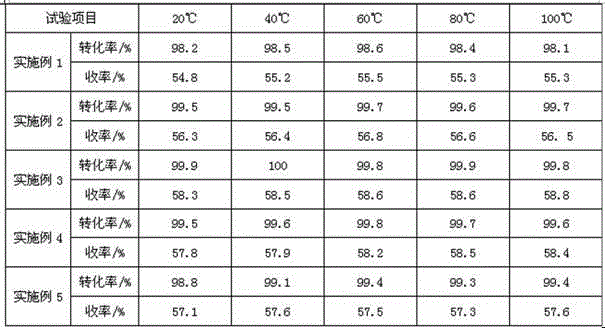
Examples
Embodiment 1
[0019] A preparation method of a catalyst for preparing difluoroethanol by hydrogenating methyl difluoroacetate, comprising the following steps:
[0020] Step 1, by weight components, 2 parts of vanadium pentoxide (particle size 100 μm) and 2 parts of NbCl 3 Add it into a sodium hydroxide solution with a mass percentage of 6%, heat it to 30°C, then add 0.8 parts of polypropylene glycol and 1 part of tetraethyl orthosilicate under stirring, continue stirring for 20 minutes after the addition, and then remove the precipitate Filter, rinse with deionized water, and dry;
[0021] Step 2, in terms of weight components, add the dried solids in step 1 together with 2 parts of potassium oxide, 0.5 parts of calcium tungstate (particle size 100 μm), 1 part of zinc oxide and 6 parts of zeolite powder (particle size 500 μm) In 10 parts of calcium chloride saturated solution, stir and mix evenly, heat up to 50°C, add 1 part of potassium permanganate solution with a concentration of 7%, stir
Embodiment 2
[0023] A preparation method of a catalyst for preparing difluoroethanol by hydrogenating methyl difluoroacetate, comprising the following steps:
[0024] Step 1, by weight components, 3 parts of vanadium pentoxide (particle size 200 μm) and 3 parts of NbCl 3 Add it into a 7% potassium hydroxide solution by mass, heat it to 33°C, then add 1 part of polypropylene glycol and 2 parts of tetraethyl orthosilicate while stirring, continue stirring for 25 minutes after the addition, and then remove the precipitate Filter, rinse with deionized water, and dry;
[0025] Step 2, in terms of weight components, add the dried solids in step 1 together with 4 parts of potassium oxide, 0.6 parts of calcium tungstate (particle size 150 μm), 3 parts of zinc oxide and 8 parts of zeolite powder (particle size 600 μm) In 12 parts of calcium chloride saturated solution, stir and mix evenly, heat up to 52°C, add 1 part of potassium permanganate solution with a concentration of 5%, stir for 1 hour, cool
Embodiment 3
[0027] A preparation method of a catalyst for preparing difluoroethanol by hydrogenating methyl difluoroacetate, comprising the following steps:
[0028] Step 1, by weight components, 4 parts of vanadium pentoxide (particle size 100 μm) and 3 parts of NbCl 3 Add it into the sodium hydroxide solution with a mass percentage of 8%, heat it to 36°C, then add 1.5 parts of polypropylene glycol and 3 parts of tetraethyl orthosilicate under stirring, continue stirring for 30 minutes after the addition, and then remove the precipitate Filter, rinse with deionized water, and dry;
[0029] Step 2, in terms of weight components, add the dried solids in step 1 together with 5 parts of potassium oxide, 0.8 parts of calcium tungstate (particle size 100 μm), 5 parts of zinc oxide and 12 parts of zeolite powder (particle size 700 μm) In 18 parts of calcium chloride saturated solution, stir and mix evenly, heat up to 60°C, add 2 parts of potassium permanganate solution with a concentration of 6%,
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap