Phosphatidyl nanometer prodrug released by enzymatic response and preparation method and application thereof
A technology of phosphatidyl nanometer and oleoyl phosphatidylcholine, which is applied in the field of biomedicine to achieve the effects of tumor inhibition, good biocompatibility and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 phosphatidyl nanometer prodrug
[0043] 1.1 Preparation of phosphatidyl doxorubicin and its micelles
[0044] (1) Dissolve 0.3g of doxorubicin in 7ml of acetic acid-sodium acetate buffer (0.2M) and 1ml of isopropanol; dissolve 0.2g of phosphatidylcholine in 5ml of n-heptane; then mix the above solutions and ultrasonically After dissolving, add 100U phospholipase D enzyme solution;
[0045] (2) React at 45°C for 8 hours in a shaker at 220rmp;
[0046] (3) repeatedly extracting the reaction solution obtained in step (2) with chloroform;
[0047] (4) The mixed solution in step (3) is separated and purified by column chromatography, the mobile phase is chloroform: dehydrated alcohol: triethylamine: water (10:11.3:11.7:2.7), and the stationary phase is silica gel powder (300-400 mesh);
[0048] (5) Rotary evaporation: the solution separated in step (4) was subjected to rotary evaporation under the condition of 30° C. for 2 to 5 hours;
[004
Embodiment 2
[0066] Example 2 Structural identification and micellar property identification of phosphatidyl nanoprodrugs
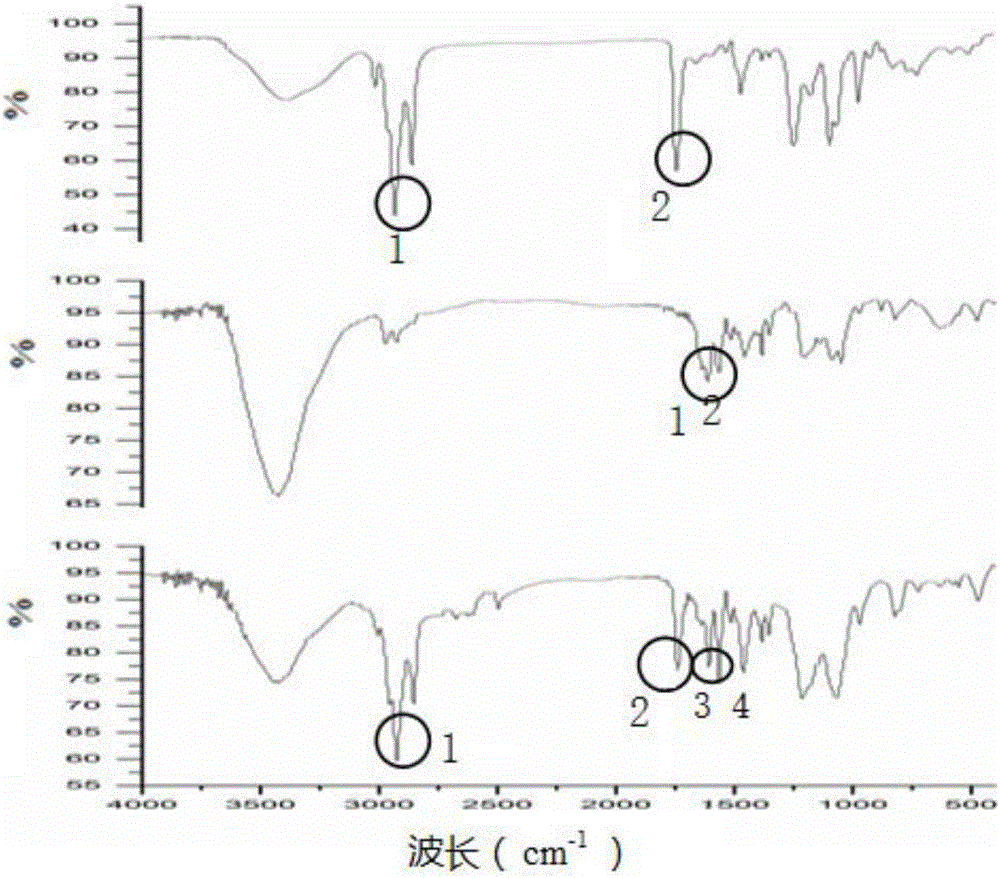
[0067] 2.1 Confirm the chemical composition of the phosphatidydoxorubicin and phosphatidymitoxantrone prepared in Example 1 by infrared method respectively, the results are as follows: Figure 1A and Figure 1B Shown: PC characteristic peak (1) 2924cm -1 , CH on the long chain 2 Stretch vibration (2) 1736.1cm -1 , The stretching peak of -C=O of -CO=O; the characteristic peak diagram of doxorubicin (A) a: (1) 1617.1cm -1 , the peak of -C=O on the benzene ring (2) 1582.6cm -1 , the stretching peak of C=C. From the infrared spectrum of phosphatidyl doxorubicin (A)c, the characteristic peaks of PC and doxorubicin can be found (1) 2924cm -1 (2)1736.1cm -1 (3)1617.1cm -1 (4)1582.6cm -1 . Also for phosphatidyl mitoxantrone, the characteristic peaks of PC and mitoxantrone can also be found.
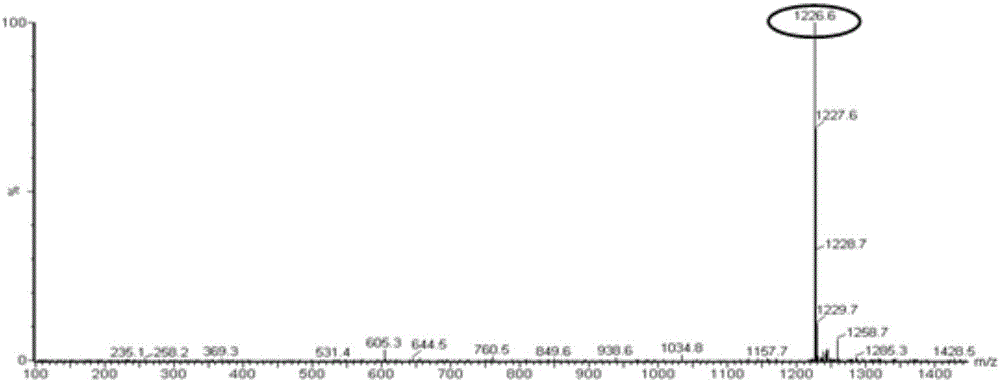
[0068] 2.2 The molecular weights of phosphatidyl doxorubicin and phosphatidy
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap