Lignanoid compound, and preparation method and application thereof
A technology for lignans and compounds, applied in the field of medicine, can solve problems such as no reports, and achieve novel structure, good research and development prospects, and the effects of preventing and treating viral infections.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation of compound of the present invention
[0033] (1) Take the finished product of Reduning Injection, separate it through HP-20 macroporous adsorption resin chromatographic column, elute with water, 25~35% ethanol, and 90~100% ethanol in sequence, collect the eluents respectively, and depressurize Concentrate until there is no alcohol smell, and obtain the water elution site, 25-30% ethanol elution site, and 90-100% ethanol elution site;
[0034] (2) Take the part eluted with 90-100% ethanol in step (1), separate it by silica gel column chromatography, and use chloroform-methanol gradient elution to collect 1.8 g of fraction B with a ratio of chloroform-methanol of 95:5. ODS column chromatography, eluted with methanol-water gradient, collected 95 mg of fraction C with a ratio of methanol-water of 6:4, fraction C was separated by semi-preparative liquid chromatography, and acetonitrile-water with a ratio of 30:70 was used as the mobile phase, the
Embodiment 2
[0035] Embodiment 2, get the compound of the present invention that embodiment 1 makes, carry out following structural identification
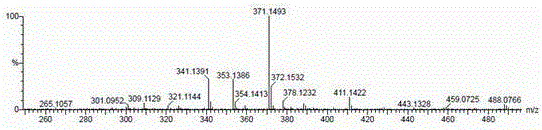
[0036] The compound is a yellow amorphous powder, ESI-MS (positive) gives m / z 411 [M+Na] + , ESI-MS(negative) gave m / z 387 [M-H] - , suggesting that the molecular weight of the compound is 388. HR-ESI-Q-TOF-MS gave m / z 411.1422 [M+Na] + (calculated value is 411.1420) (see figure 1 ), confirm that the molecular formula of the compound is C 21 h 24 o 7 , with a calculated unsaturation of 10.
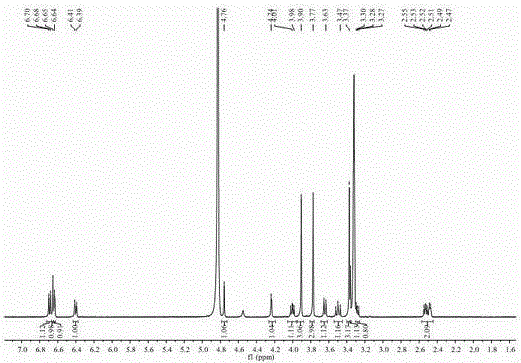
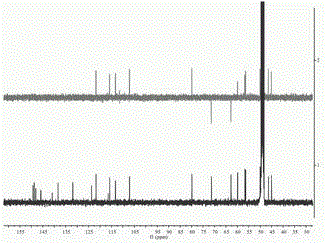
[0037] of this compound 1 H-NMR (400 MHz, in CD 3 OD) spectrum (see figure 2 ), showing 21 hydrogen signals, 4 aromatic hydrogen signals [ δ 6.65 (1H, s, H-2′), 6.64 (1H, d, J =1.6 Hz, H-2), 6.69 (1H,d, J = 8.0 Hz, H-5), 6.40 (1H, dd, J [ δ 3.90 (3H, s), 3.37 (3H, s), 3.77 (3H, s)], suggesting that it may be connected to the benzene ring. 13 C-NMR (100MHz, in CD 3 OD) combined with DEPT-135 profile (see image 3 ) showed a to
Embodiment 3
[0042] Example 3, the compounds of the present invention are tested against H1N1 and H3N2 in vitro
[0043] 1. Materials
[0044] 1.1 Virus strains Influenza A virus (A / Human / Hubei / 2005 H3N2) and influenza A virus (A / PR / 8 / 34H1N1), passaged and preserved by Wuhan Institute of Virology, Chinese Academy of Sciences;
[0045] 1.2 Cell model The dog kidney cell line MDCK was preserved in our laboratory. Culture conditions: DMEM+10% fetal bovine serum, 37°C, 5% CO2;
[0046] 2. Principles and methods
[0047] 2.1 Drug cytotoxicity detection
[0048] Assay principle: alamarBlue® is a redox indicator that produces an absorbance change and a fluorescent signal according to metabolic activity. AlamarBlue® is easily soluble in water, and its oxidized form enters the cells and is reduced by mitochondrial enzymes to produce measurable fluorescence and color changes, which are used for quantitative analysis of cell viability and cell proliferation and in vitro cytotoxicity stud
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap