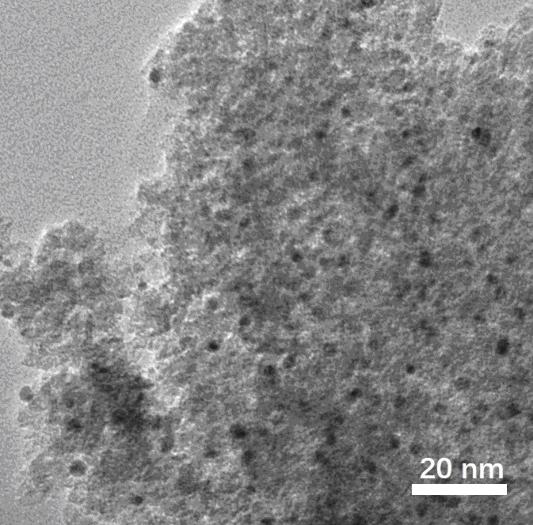
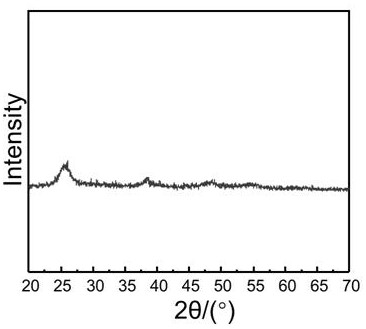
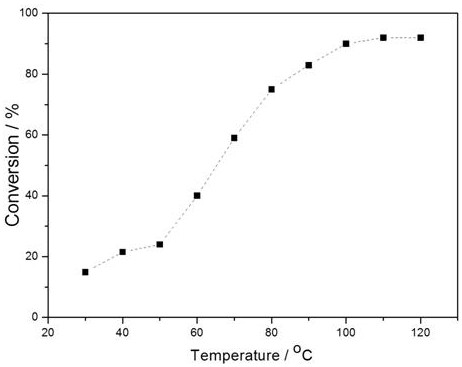
A kind of preparation method of gold palladium nano-catalyst in CO oxidation reaction
A nano-catalyst, oxidation reaction technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. , loss and other problems, to achieve the effect of overcoming the agglomeration of catalyst particles, high activity and sufficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 100mg of titanium dioxide carrier, 5mL molar concentration of 2.0mmol L -1 HAuCl 4 Precursor solution and 5 mL molar concentration of 2.0 mmol L -1 H 2 PdCl 4 The precursor solution was mixed evenly, stirred and reacted at 25°C for 4h, and then the mixture was separated by centrifugation, and the catalyst was washed with deionized water to remove chloride ions and other impurities on the surface, and then dried in an oven at 50°C. The dried sample was placed in an inert atmosphere The gold-palladium nano-catalyst was prepared by heat treatment at 300°C, and then naturally cooled down to prepare the gold-palladium nano-catalyst. In the CO oxidation reaction, the conversion rate of CO was 90% at 100°C.
Embodiment 2
[0018] 100mg of titania support, 4mL molar concentration of 2.0mmol L -1 HAuCl 4 Precursor solution and 6 mL molar concentration of 2.0 mmol L -1 H 2 PdCl 4 The precursor solution was mixed evenly, stirred and reacted at 25°C for 4h, and then the mixture was separated by centrifugation, and the catalyst was washed with deionized water to remove chloride ions and other impurities on the surface, and then dried in an oven at 50°C. The dried sample was placed in an inert atmosphere The gold-palladium nano-catalyst was prepared by heat treatment at 300°C, and then naturally lowered the temperature. In the CO oxidation reaction, the conversion rate of CO was 84% at 100°C.
Embodiment 3
[0020] 100mg of titanium dioxide carrier, 6mL molar concentration of 2.0mmol L -1 HAuCl 4 Precursor solution and 4 mL molar concentration of 2.0 mmol L -1 H 2 PdCl 4 The precursor solution was mixed evenly, stirred and reacted at 25°C for 4h, and then the mixture was separated by centrifugation, and the catalyst was washed with deionized water to remove chloride ions and other impurities on the surface, and then dried in an oven at 50°C. The dried sample was placed in an inert atmosphere The gold-palladium nano-catalyst was prepared by heat treatment at 300°C, and then naturally lowered the temperature. In the CO oxidation reaction, the conversion rate of CO was 75% at 100°C.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap