Method for preparing cyclic phosphate or cyclic phosphite
A technology of cyclic phosphite and cyclic phosphate, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as complex process routes and difficult handling of hydrogen chloride, Achieve the effect of simple process, low cost and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
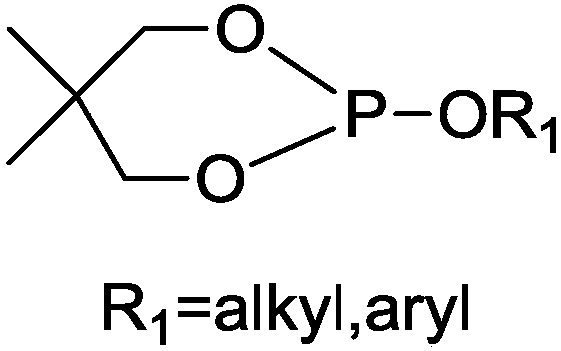
[0025] Add 0.1 mol of phosphoric acid triester and 0.13 mol of neopentyl glycol into a four-necked flask equipped with a thermometer, an electric stirrer, and a reflux condenser, wherein the mass of 0.13 mol of neopentyl glycol is 13.52 g, and the temperature is raised to 140 ° C. Then add 0.068g of catalyst triethylamine, keep warm at 140-150°C for 9h; collect by-products, and distill off unreacted substances under reduced pressure after the reaction to obtain a light yellow liquid product with a yield of 81.3%.
[0026] The structural formula of the product obtained in the present embodiment is as follows:
[0027]
Embodiment 2
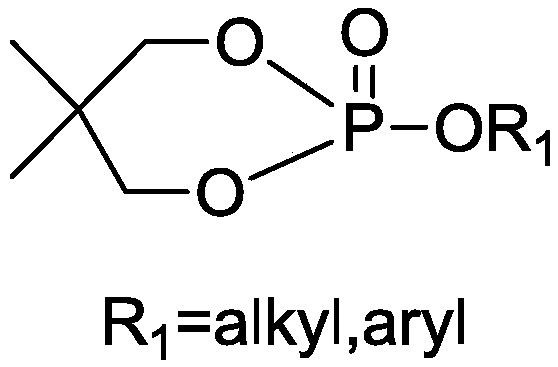
[0029] The 0.1 mol phosphotriester in Example 1 was replaced with 0.1 mol phosphite triester, and the others were the same as in Example 1, and the yield was 81.3%.
[0030] The structural formula of the product obtained in the present embodiment is as follows:
[0031]
Embodiment 3
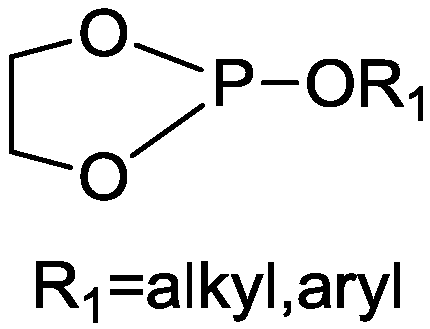
[0033] Add 0.1moL of phosphoric acid triester and 0.10moL of ethylene glycol into a four-neck flask equipped with a thermometer, an electric stirrer, and a reflux condenser. Among them, the mass of 0.10moL of ethylene glycol is 6.21g, raise the temperature to 110°C, and then add Catalyst triethylamine 0.062g, heat preservation reaction at 110-130° C. for 5 hours, collect by-products, remove unreacted substances by vacuum distillation after the reaction, and obtain a light yellow liquid product with a yield of 81.3%.
[0034] The structural formula of the product obtained in the present embodiment is as follows:
[0035]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap