Dual agonist peptide of adiponectin receptor-1 and receptor-2 for treating NAFLD and liver fibrosis
A dual agonist and adiponectin technology, applied in the field of biochemistry, can solve the problems of no peptide drug development and achieve good biological activity, low toxicity, and large safety window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the synthesis of double agonist peptide
[0054] Materials: All amino acids were purchased from NovaBiochem;
[0055] Unless otherwise specified, all other reagents were of analytical grade and purchased from Sigma;
[0056] Using Protein Technologies PRELUDE 6-channel synthetic peptide synthesizer;
[0057] Phenomenex Luna C18 preparative column (46mm x 250mm) was used to purify synthetic peptides;
[0058] The high-performance liquid chromatograph is a product of Waters;
[0059] Mass spectrometry was performed using an Agilent mass spectrometer.
[0060] The compound Pep70 (structural sequence: PGLYYFD-NH2) is taken as an example to illustrate the synthesis method of the dual agonist peptide of the present invention.
[0061] a) Assembly of the main peptide chain:
[0062] According to the Fmoc / t-Bu strategy, the following synthetic peptides were synthesized on a CS336X synthetic peptide synthesizer (CS Bio, USA) at a scale of 0.25 mmol: Pro-Gly-Leu
Embodiment 2
[0071] Example 2, Cytotoxicity evaluation using the dual agonist peptide obtained in Example 1 as a test substance
[0072] Human hepatic stellate cell LX2 cell line was selected to study and observe the detection of the test substance on the proliferation and toxicity of LX2 cells.
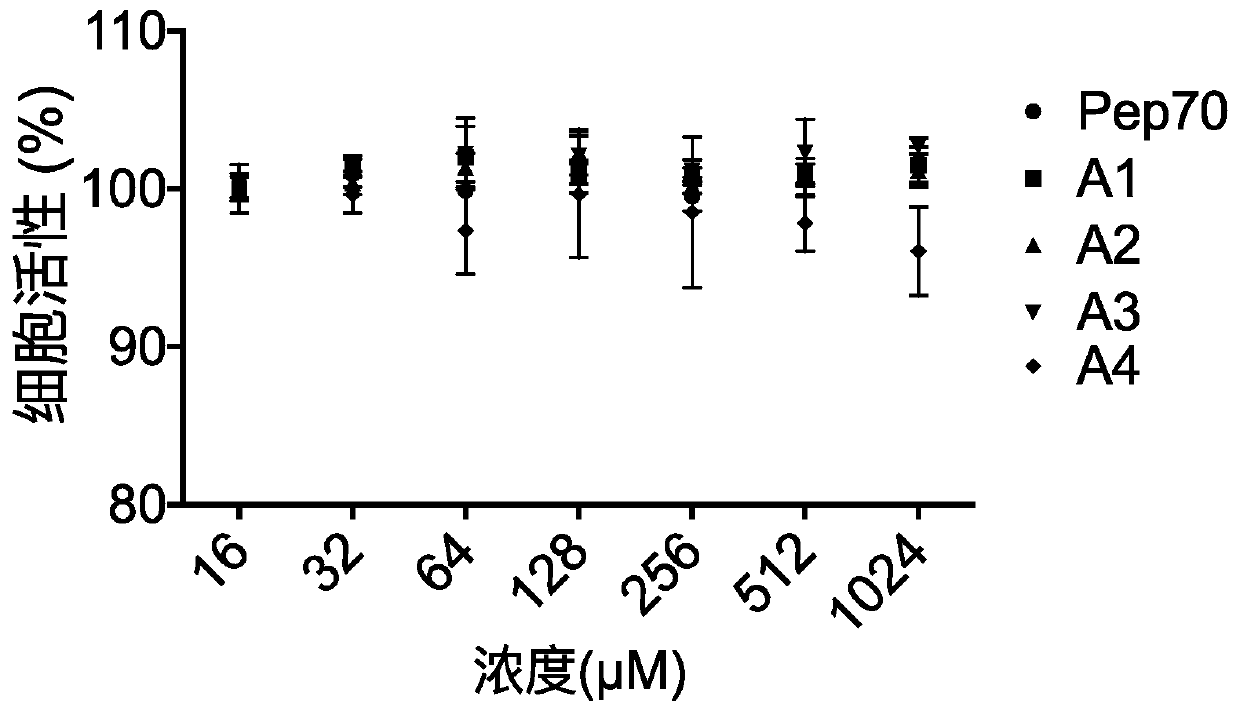
[0073] LX2 cells were inoculated into 96-well plates and cultured with DMEM (high glucose) + 10% FBS (fetal bovine serum) + 1% double antibody medium (Thermo Fisher) at 37°C, 5% CO 2 When the cells grew to 70% confluence under the condition, different concentrations of test substances were added, and after incubation for 48 hours, CCK8 reagent was added, and the absorbance was detected at a wavelength of 450 nm.
[0074] figure 1 is the percentage of cell viability in each group, figure 1 It shows that in the concentration range of 0-1024μM, it shows that the tested cells maintain a high and basically the same level of cell activity, and the cell activity is not affected, indicating that the test
Embodiment 3
[0075] Example 3. Using the Pep70 obtained in Example 1 and the dual agonist peptides A1-A4 as test substances to carry out the in vitro inhibitory effect experiment on fatty liver
[0076] The human hepatocellular carcinoma HepG2 cell line was selected to study and observe the improvement of the fat accumulation of HepG2 cells induced by palmitic acid (PA).
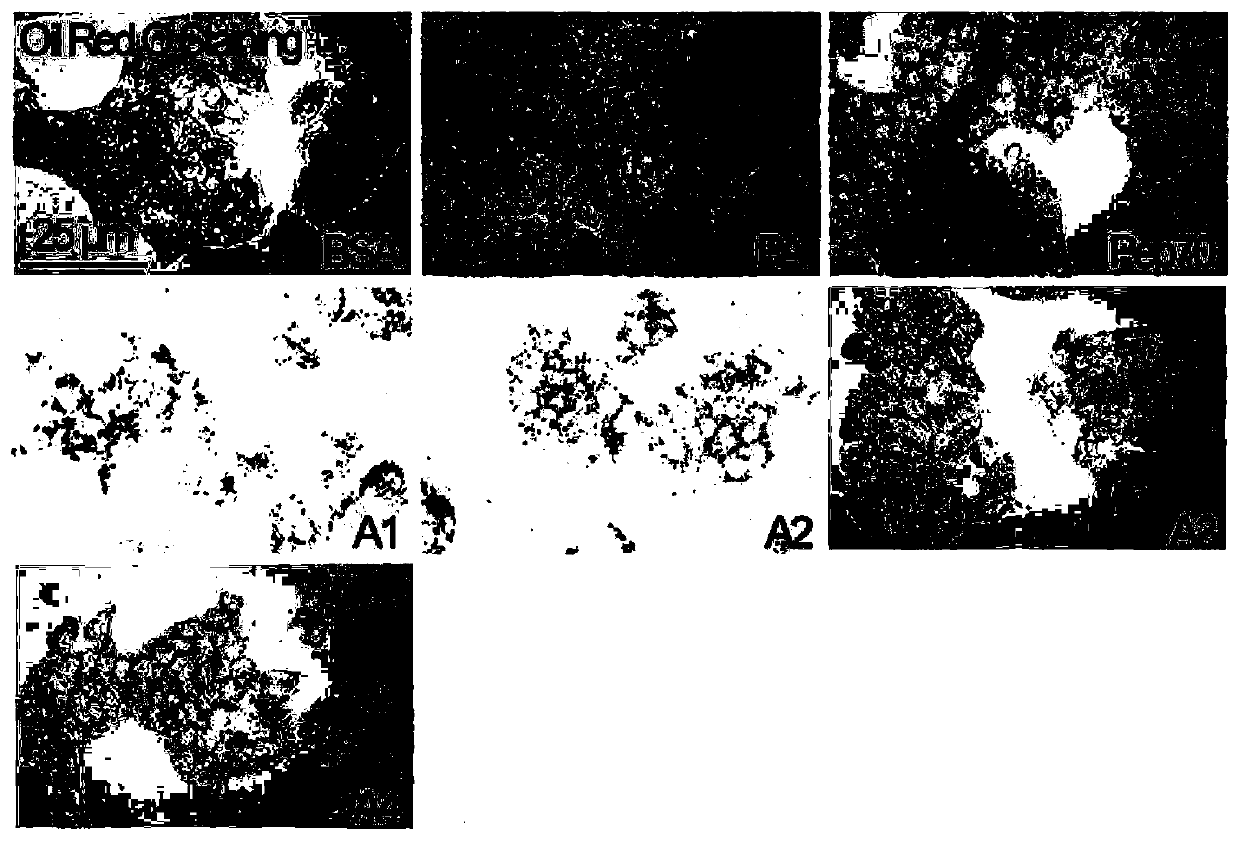
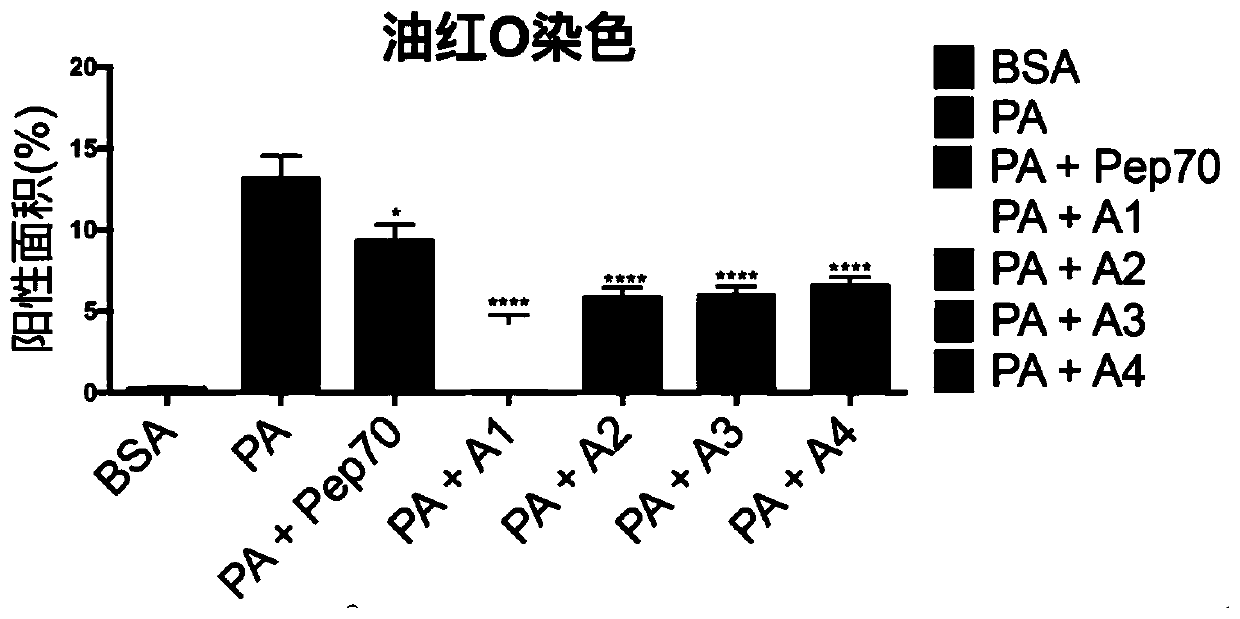
[0077] HepG2 cells were plated in 6-well plates, cultured with DMEM (high glucose) + 10% FBS + 1% double antibody medium (ThermoFisher), at 37°C, 5% CO 2 When the cells grew to 70% confluency under the same conditions, HepG2 cells were induced with palmitic acid (PA) at a final concentration of 0.25 mM for 24 h, and then 100 μM of the test substance was added, and the same volume of DMEM was added to the control group for 24 h. , Oil red O staining was performed to detect the effect of drugs on fatty degeneration cells.
[0078] figure 2 Oil red O staining photos of the test substance and the control group, the stained a
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap