Long-cycle high-rate aqueous sodium-manganese battery
A sodium-manganese battery, high-rate technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problem of water-based sodium-ion battery rate performance, energy density and cycle life that do not meet the requirements, low battery voltage and capacity, rate and Problems such as poor cycle performance, to achieve the effects of high rate performance and cycle stability, adjustable capacity, and high rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
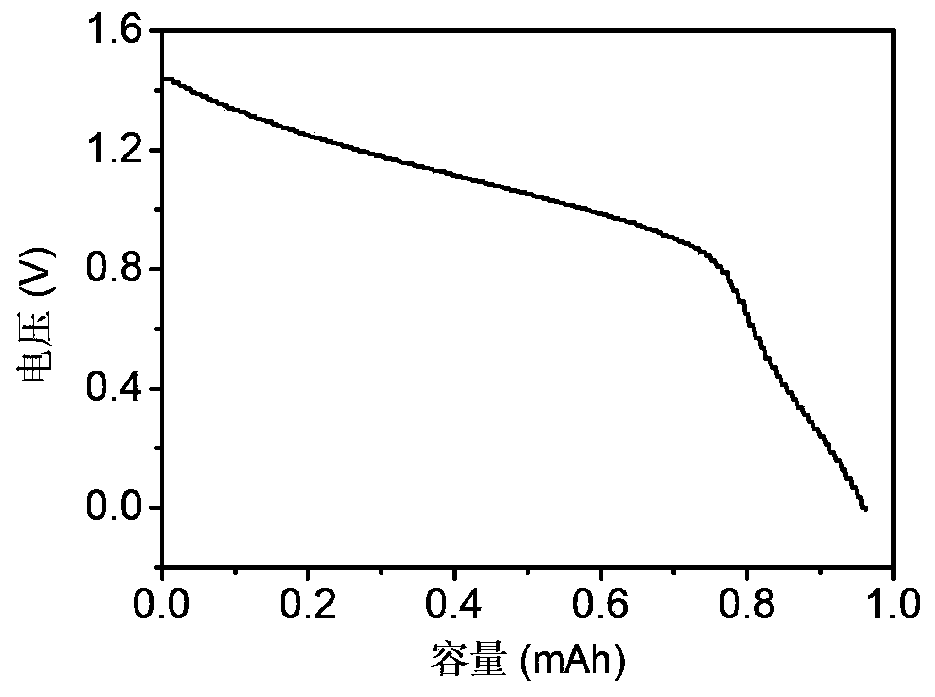
[0015] This embodiment provides an aqueous sodium manganese battery, including a positive electrode, a negative electrode, a mixed electrolyte and a separator. The positive electrode is a graphite felt matrix, the negative electrode is activated carbon, and the electrolyte is MnSO. 4 +Na 2 SO 4 / H 2 SO 4 , The diaphragm is filter paper.
[0016] First, the cathode graphite felt matrix is processed. The graphite felt substrate was soaked in acetone overnight, and then ultrasonically washed with ultrapure water and ethanol. Then, the graphite felt was added to the sulfuric acid solution in which ammonium persulfate was dissolved, and ultrasonically stirred for 15 minutes. The ultrasonic graphite felt was washed with water and ethanol, dried, and calcined in a muffle furnace at 450°C for 30 minutes.
[0017] The negative electrode includes a current collector and a negative electrode material attached to the current collector. The preparation method is to uniformly mix the activated
Embodiment 2
[0022] The difference is that the anode material includes activated carbon powder, Super P and sodium carboxymethyl cellulose, and the mass ratio of activated carbon powder, SuperP and sodium carboxymethyl cellulose is 8:1:1.
[0023] The electrolyte is Mn(NO 3 ) 2 +NaNO 3 / H 2 SO 4 , Mn(NO 3 ) 2 And NaNO 3 The concentration is 1mol / L, H 2 SO 4 The concentration is 0.1mol / L.
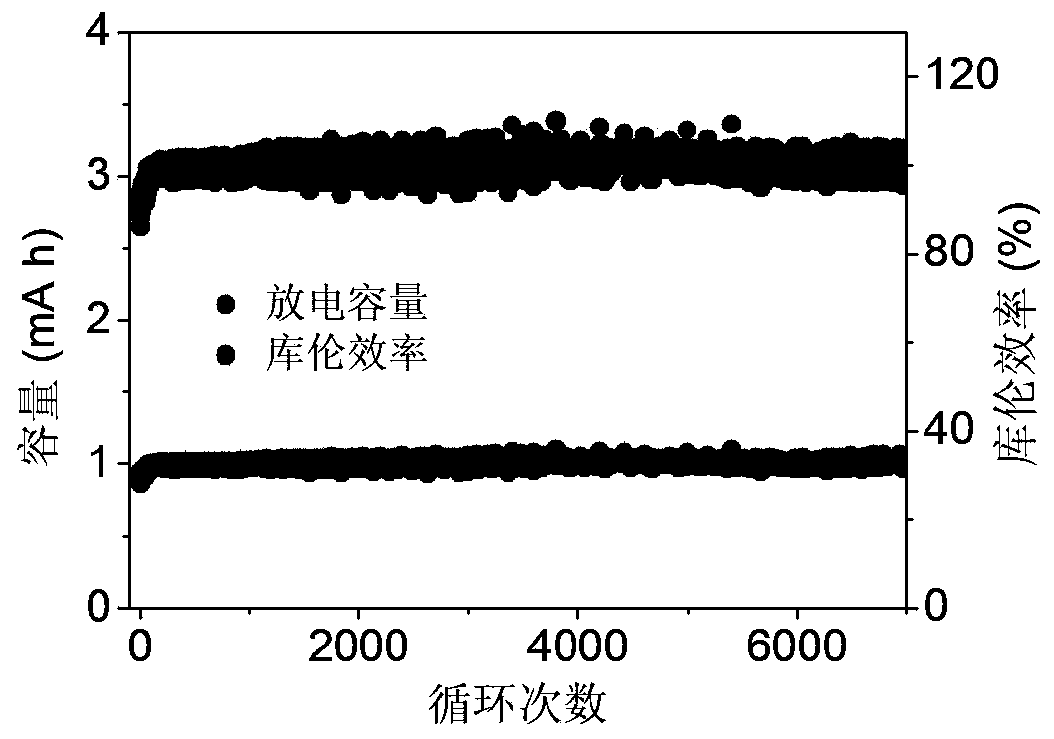
[0024] image 3 To charge at 1.5V constant voltage, the charge cut-off capacity is 1mAh cm -2 ; With 10mA cm -2 The cycle performance graph of the battery system under constant current discharge and the discharge cut-off voltage of 0V. It can be seen that the efficiency of the battery with more than 6000 cycles has been close to 100%, the capacity has not declined, and it has very good cycle stability.
[0025] Figure 4 It is the rate performance graph of the battery system in Example 2. It is charged at a constant voltage of 1.5V, and the charge cut-off capacity is 1mA hcm -2 , Respectively with 10, 20, 50
Embodiment 3
[0027] The difference is that the negative electrode material includes activated carbon powder, Ketjen black and polytetrafluoroethylene, and the mass ratio of activated carbon powder, Ketjen black and polytetrafluoroethylene is 8:1:1. The negative electrode current collector is carbon paper.
[0028] Electrolyte is MnSO 4 +Na 2 SO 4 / H 2 SO 4 , MnSO 4 And Na 2 SO 4 The concentration is 1mol / L, H 2 SO 4 The concentration is 0.3mol / L.
[0029] Figure 5 Is the battery system in Example 3 under different charging capacities, 10mA cm -2 Diagram of capacity storage capacity discharged under current density. Charge with 1.5V constant voltage, charge cut-off capacity is 1, 2, 3, 4, 5mA h cm -2 , Each cycle 10 times; with 10mA cm -2 The discharge current is constant current, and the discharge cut-off voltage is 0V. It can be seen that when the charging capacity is increased from 1 mA h to 5 mA h, the coulombic efficiency of the battery hardly decays, close to 100%, showing a good adj
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap