Imidazole triazine isocyanuric acid latent curing agent as well as synthesis method and application thereof
A technology of latent curing agent, imidazolium triazine isocyanate, which is applied in the field of new imidazolium triazine isocyanuric acid latent curing agent and its synthesis, can solve the problems of short pot life and achieve high safety in use, stable performance, Apply a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Synthesis of 2,4-diamino-6-[2-(2-methyl-1-imidazole) ethyl]-1,3,5-triazine isocyanuric acid
[0054] Add 2,4-diamino-6-[2-(2-methyl-1-imidazolium)ethyl]-1,3,5-triazine (1mol) into a 250mL circular cylinder with a stirring and condensing reflux device In a bottom flask, add isocyanuric acid (1mol), add ethanol (100mL), and stir the reaction at 80°C for 5 hours. When the residual amount of 2,4-diamino-6-[2-(2-methyl-1-imidazole)ethyl]-1,3,5-triazine detected by liquid chromatography is ≤0.05%, it is confirmed that after the reaction , centrifuged to remove the solvent, and dried to obtain the target product (white solid) - 2,4-diamino-6-[2-(2-methyl-1-imidazole) ethyl]-1,3,5 - Triazine isocyanuric acid, the yield of which is 92%. The synthetic reaction formula is as follows formula IV:
[0055]
[0056] Among them, R 1 is methyl, R 2 for hydrogen.
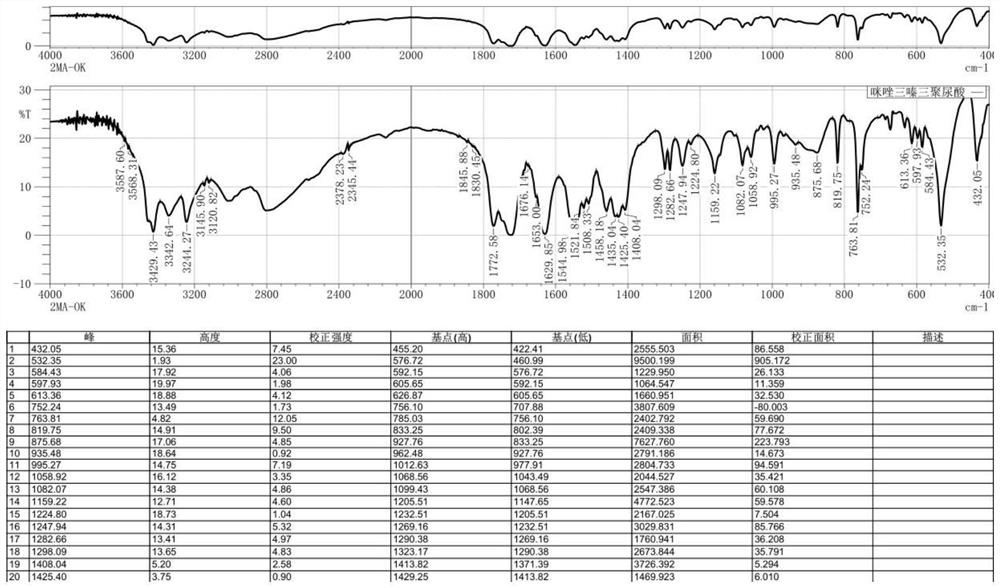
[0057] The IR data of the product 2,4-diamino-6-[2-(2-methyl-1-imidazole) ethyl]-1,3,5-triazine isocyanuric
Embodiment 2
[0064] Example 2: Synthesis of 2,4-diamino-6-[2-(2-phenyl-1-imidazole) ethyl]-1,3,5-triazine isocyanuric acid
[0065] Add 2,4-diamino-6-[2-(2-phenyl-1-imidazole)ethyl]-1,3,5-triazine (1mol) into a 250mL round bottom flask, and add iso Add methanol (100 mL) to cyanuric acid (1 mol), and stir the reaction at 80°C for 5 hours. When the residual amount of 2,4-diamino-6-[2-(2-phenyl-1-imidazole)ethyl]-1,3,5-triazine detected by liquid chromatography is ≤0.05%, it is confirmed that after the reaction , centrifuged to remove the solvent, and dried to obtain the target product (white solid) - 2,4-diamino-6-[2-(2-phenyl-1-imidazole) ethyl]-1,3,5 - Triazine isocyanuric acid, the yield of which is 98%.
Embodiment 3
[0066] Example 3: Synthesis of 2,4-diamino-6-[2-(2-isopropyl-1-imidazole) ethyl]-1,3,5-triazine isocyanuric acid
[0067] Add 2,4-diamino-6-[2-(2-isopropyl-1-imidazole)ethyl]-1,3,5-triazine (1mol) into a 250mL round bottom flask, and add Isocyanuric acid (1mol), deionized water (100mL) was added, and the reaction was stirred at 110°C for 4 hours. When the residual amount of 2,4-diamino-6-[2-(2-isopropyl-1-imidazole)ethyl]-1,3,5-triazine is detected by liquid chromatography ≤0.05%, it is confirmed that the reaction is complete Afterwards, centrifugal dehydration, drying to obtain the target product (white solid) - 2,4-diamino-6-[2-(2-isopropyl-1-imidazole) ethyl]-1,3,5 - Triazine isocyanuric acid, the yield of which is 99%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap