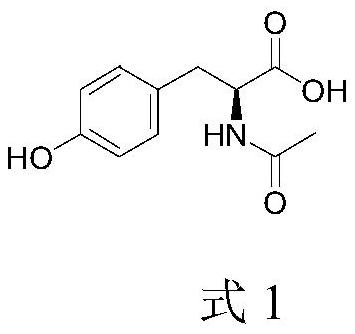
Method for preparing N-acetyl-L-tyrosine
A technology of tyrosine and acetyl, applied in the field of preparing N-acetyl-L-tyrosine, which can solve the problems of high substance content, high production cost, and large energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
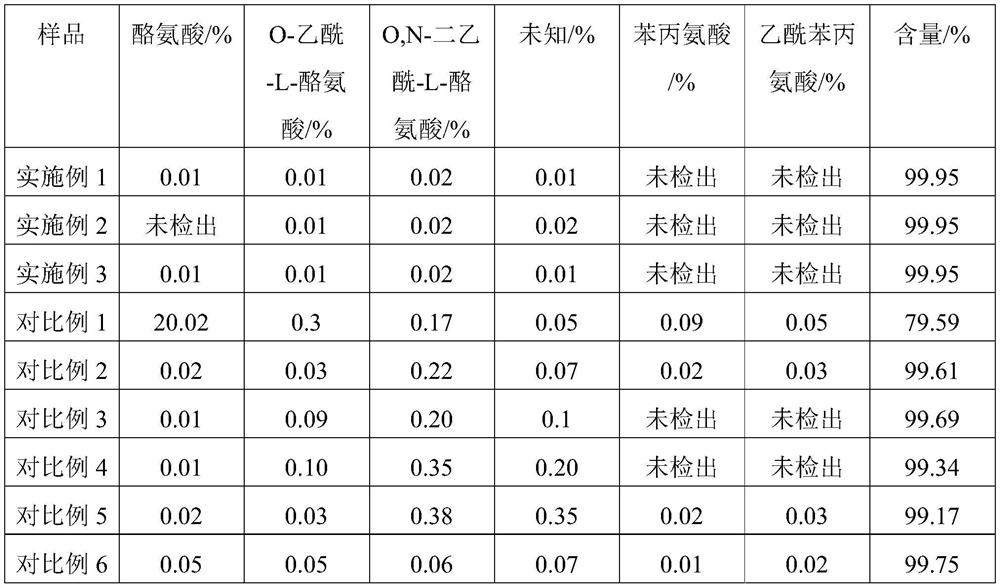
Examples
Embodiment 1
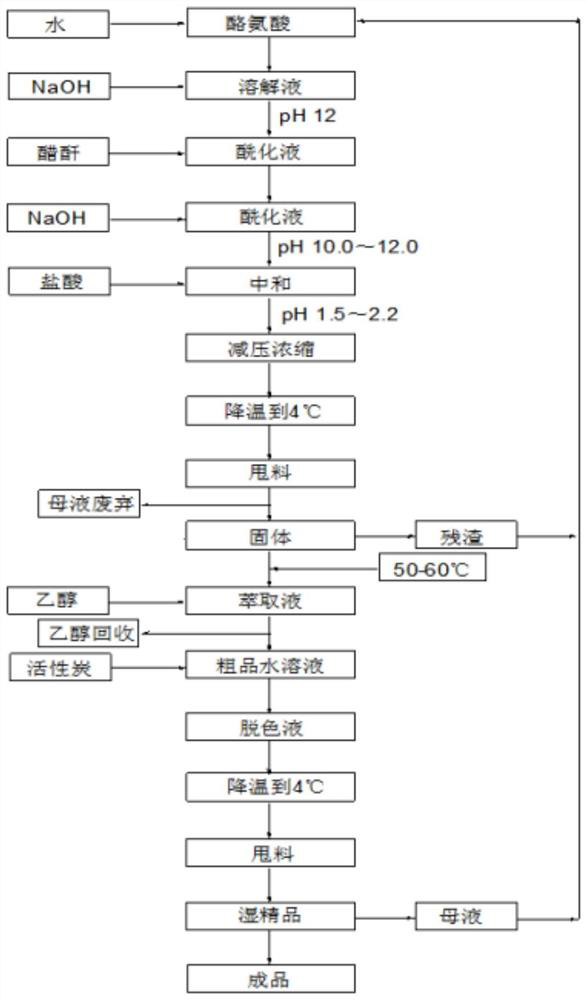
[0036] In this example, N-acetyl-L-tyrosine was prepared according to the following steps, and the process flow diagram is shown in figure 1 :
[0037] Step 1. Disperse 100 g of L-tyrosine in 200 ml of water, stir quickly to avoid agglomeration.
[0038] Step 2. Add 30% sodium hydroxide solution dropwise until the L-tyrosine is completely dissolved, pH=12.05.
[0039] Step 3. Add 59.2 g (1.05 times the molar amount) of acetic anhydride dropwise for 30 minutes, while adding 30% sodium hydroxide solution dropwise to maintain the pH between 8 and 10.
[0040] Step 4. After adding acetic anhydride, dropwise add sodium hydroxide solution to adjust pH=11.50, and keep the temperature at 60°C for 20 minutes.
[0041] Step 5. Add industrial hydrochloric acid to adjust the pH of the solution to 1.72.
[0042] Step 6, control the vacuum to be above 0.08MP, and concentrate under reduced pressure at 80°C until the reaction solution is basically in a solid state.
[0043] Step 7, after bei
Embodiment 2
[0050] In this example, N-acetyl-L-tyrosine was prepared as follows:
[0051] Step 1. Disperse 500 grams of L-tyrosine in 1 liter of water and stir quickly to avoid agglomeration.
[0052] Step 2. Add 30% sodium hydroxide solution dropwise until the L-tyrosine is completely dissolved, pH=11.80.
[0053] Step 3. Add 296.3 g (1.05 times the molar amount) of acetic anhydride dropwise for 30 minutes, while adding 30% sodium hydroxide solution dropwise to maintain the pH between 8 and 10.
[0054] Step 4. After adding acetic anhydride, adjust pH=11.22 with sodium hydroxide solution, and keep the temperature at 60°C for 20 minutes.
[0055] Step 5. Add industrial hydrochloric acid to adjust the pH of the solution to 1.90.
[0056] Step 6, control the vacuum to be above 0.08MP, and concentrate under reduced pressure at 60-80°C until the reaction solution is basically in a solid state.
[0057] Step 7. After cooling to 4°C for full crystallization, the material is thrown away to obtai
Embodiment 3
[0063] In this example, N-acetyl-L-tyrosine was prepared as follows:
[0064] Step 1. Disperse 100 grams of L-tyrosine in 100 ml of water, stir quickly to avoid agglomeration.
[0065] Step 2. Put the high-quality mother liquor of Example 1 and the washed insoluble matter (L-tyrosine) into the reaction vessel together.
[0066] Step 3. Add 30% sodium hydroxide solution dropwise until L-tyrosine is completely dissolved, pH=12.25.
[0067] Step 4. Add 59.2 grams (1.05 times the molar amount) of acetic anhydride dropwise for 30 minutes, while adding 30% sodium hydroxide solution dropwise to maintain the pH between 8 and 10.
[0068] Step 5. After adding acetic anhydride, adjust pH=10.99 with sodium hydroxide solution, and keep the temperature at 60°C for 30 minutes.
[0069] Step 6, adding industrial hydrochloric acid to adjust the pH of the solution to 1.87.
[0070] Step 7, control the vacuum to be above 0.08MP, and concentrate under reduced pressure at 80°C until the reaction
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap