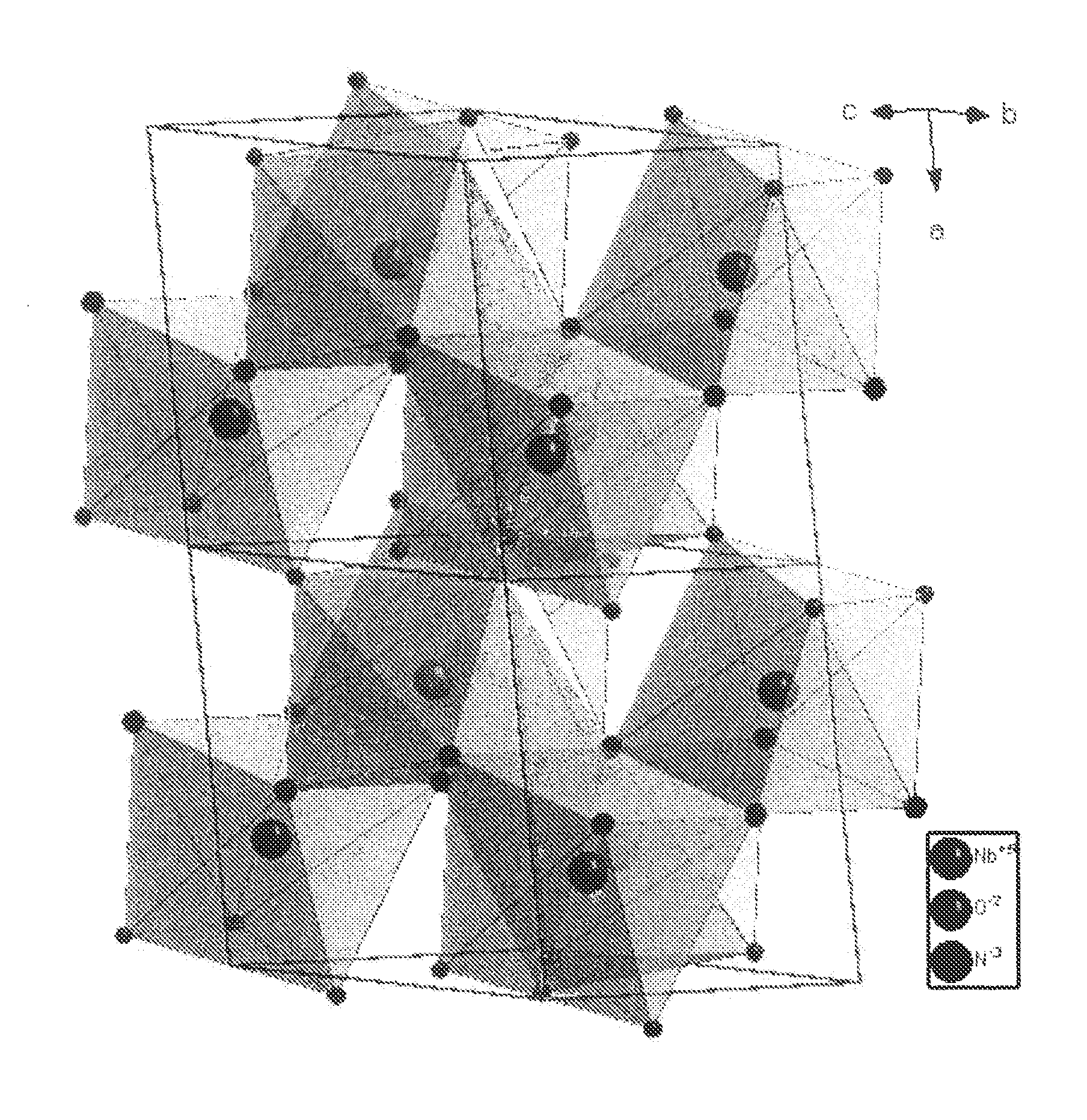
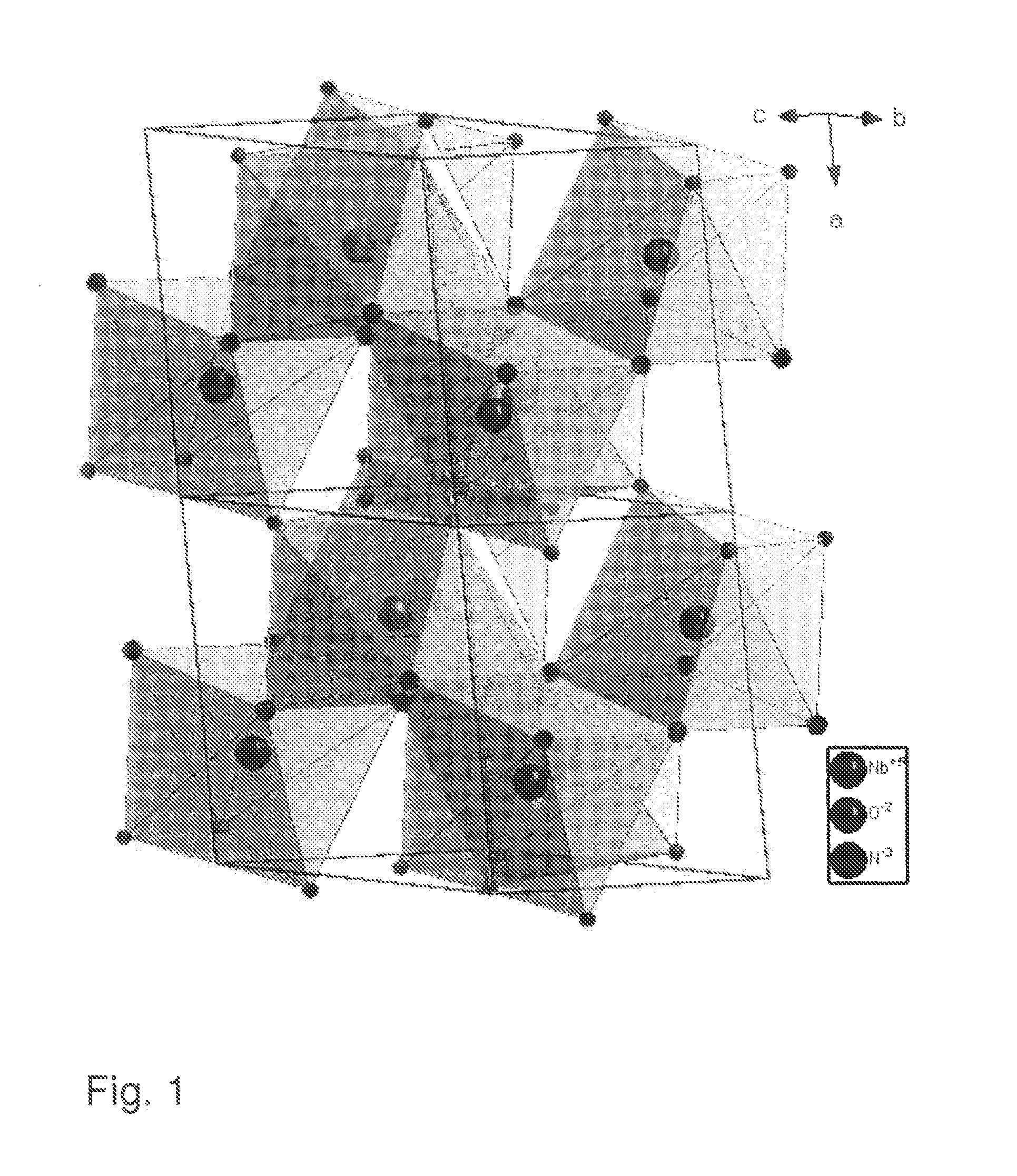
Transition metal oxidenitrides
a technology of oxidenitrides and transition metals, which is applied in the direction of electrochemical generators, cell components, electrochemical generators, etc., can solve the problems of reducing the electric and li-ion conductivity of oxides and phosphates, limiting the charge/discharge speed, and reducing the weight of such products, so as to enhance the electric performance of nanoparticles and the effect of conductive coatings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070]Ammolysis of Transition Metal Oxides
[0071]Preferred starting electrode materials are MoO3, V2O5, CrO3, WO3, TiO2, MnO2, NiO2, CoO2, more particularly MoO3. As we mentioned in the background section, MoO3 has been considering a potential electrode material for many years because of its attractive Mo6+ / Mo4+ redox couple. Capacity of electrodes made of MoO3 nanofibers can reach as high as 350 mAh / g in the first discharge, which is near to the theoretical capacity of MoO3, 380 mAh / g (insertion of 2 Li+ per Mo atom). However, after the first cycle, capacity decreases dramatically. Herein, we further describe the nitrogen doped MoO3 and the influence of doping on electrochemical performance.
[0072]1. Synthesis of MoO3 Nanofibers
[0073]The synthesis of nanofibers of MoO3 was reported elsewhere43. Typical reaction is, gently putting grams of molybdenum powder into 20 ml H2O2 (30%) to obtain yellowish MoO2(OH)(OOH) solution in a water-ice bath, loading the solution into a 40 ml Teflon
example 2
Ammolysis of Lithium Containing Transition Metal Oxides
[0088]This nitrogen doping process can also be used in lithium containing compounds successfully. Preferred starting electrode materials are LixMoOy, LixV2Oy, LixCrOy, LixWOy, LixTiOy, LixMnOy, LixNiOy, LixCoOy, more particularly Li2MoO4.
[0089]1. Nitrogen Doping of Lithium Molybdates
[0090]Herein, ammolysis of Li2MoO4 is taken as an example. The starting material is commercial chemical Li2MoO4 from Alfa Aesar (99+% purity). Table 2 lists the products obtained from ammolysis of Li2MoO4 under different temperature programs.
TABLE 2Temperature programs and products for ammolysis of Li2MoO4No.Temperature programProducts (from XRD)A450° C. / 10 hoursLi2MoO4B480° C. / 10 hoursLi2MoO4 with peak shiftsC500° C. / 10 hoursγ-Mo2N, Li2MoO4, unknown phaseD520° C. / 10 hoursγ-Mo2N, Li2MoO4, unknown phaseE550° C. / 10 hoursγ-Mo2N, unknown phaseF600° C. / 10 hoursγ-Mo2N, unknown phase
[0091]Li2MoO4 is water soluble, white color and an insulator compo
example 3
[0093]Nitridation Followed by Selective Oxidation
[0094]3.1Materials and Methods
[0095]3.1.1 Chemicals and Synthesis Methods
[0096]NbOCl3 was prepared by gas transport reaction. Nb2O5 (>99%, JMC) and NbCl5 (99.8%, Acros) were mixed in molar ratio 1:3, sealed in Pyrex tube and heated up to 400° C. in 4 hours and keep at this temperature for 40 hours. Deep green needle-shaped crystals of NbOCl3 were produced. These were then ground into a fine white powder and reacted with ammonia at room temperature until the color changed into bright yellowish niobium oxychloride amide NbOCl3(NH3)x. To make sure the reaction was carried out completely, a second grinding was necessary. Considering that NbCl5 and NbOCl3 are both water sensitive, the experiments were carried out in a glove box under protective Ar atmosphere.
[0097]NbNO was synthesized by decomposition of niobium oxychloride amide. In a typical reaction, 0.8 g NbOCl3(NH3)x were sealed in a Pyrex tube (8 mm inner diameter and 1 m length) which
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap