Purification of Organic Compounds Using Surrogate Stationary Phases on Reversed Phase Columns
a technology of organic compounds and stationary phases, which is applied in the direction of carrier-bound/immobilised peptides, cation exchangers, component separation, etc., can solve the problems of limited quantity, limited quantity, and high cost of large-scale hplc instruments and associated column hardware, so as to reduce waste disposal, increase loading, and limit the use of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example-1
Preparative RP-HPLC of Leuprolide Acetate
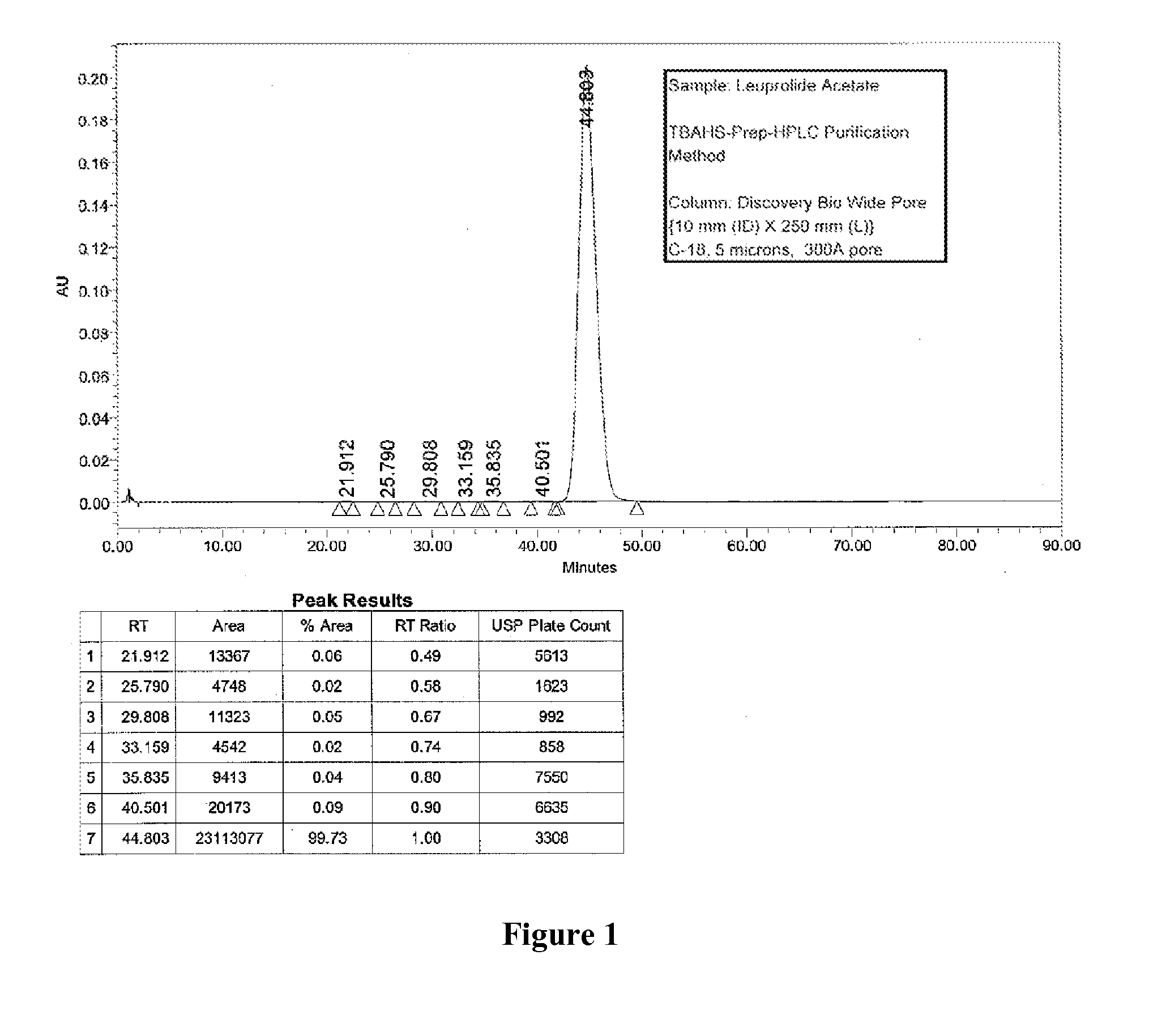
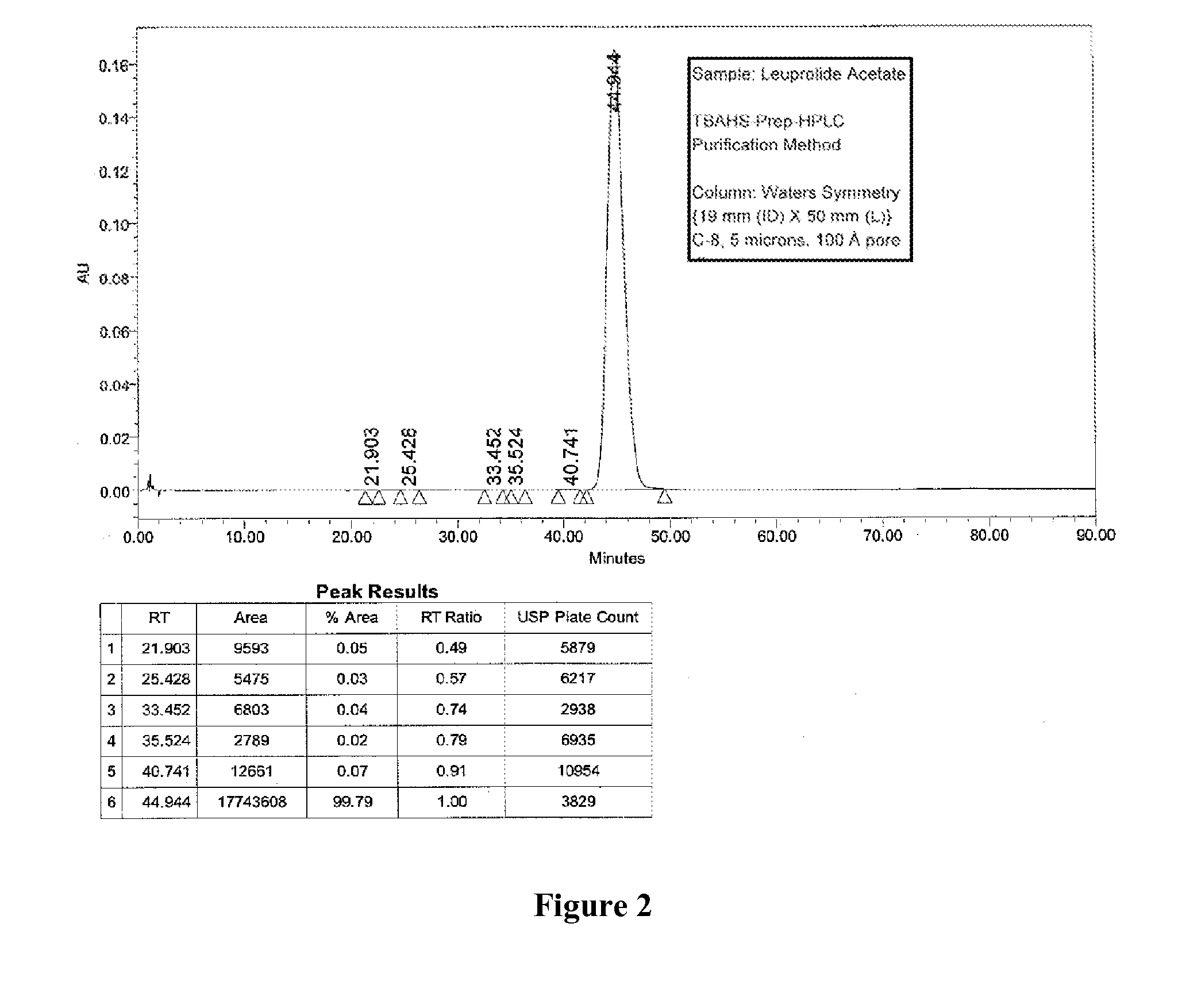
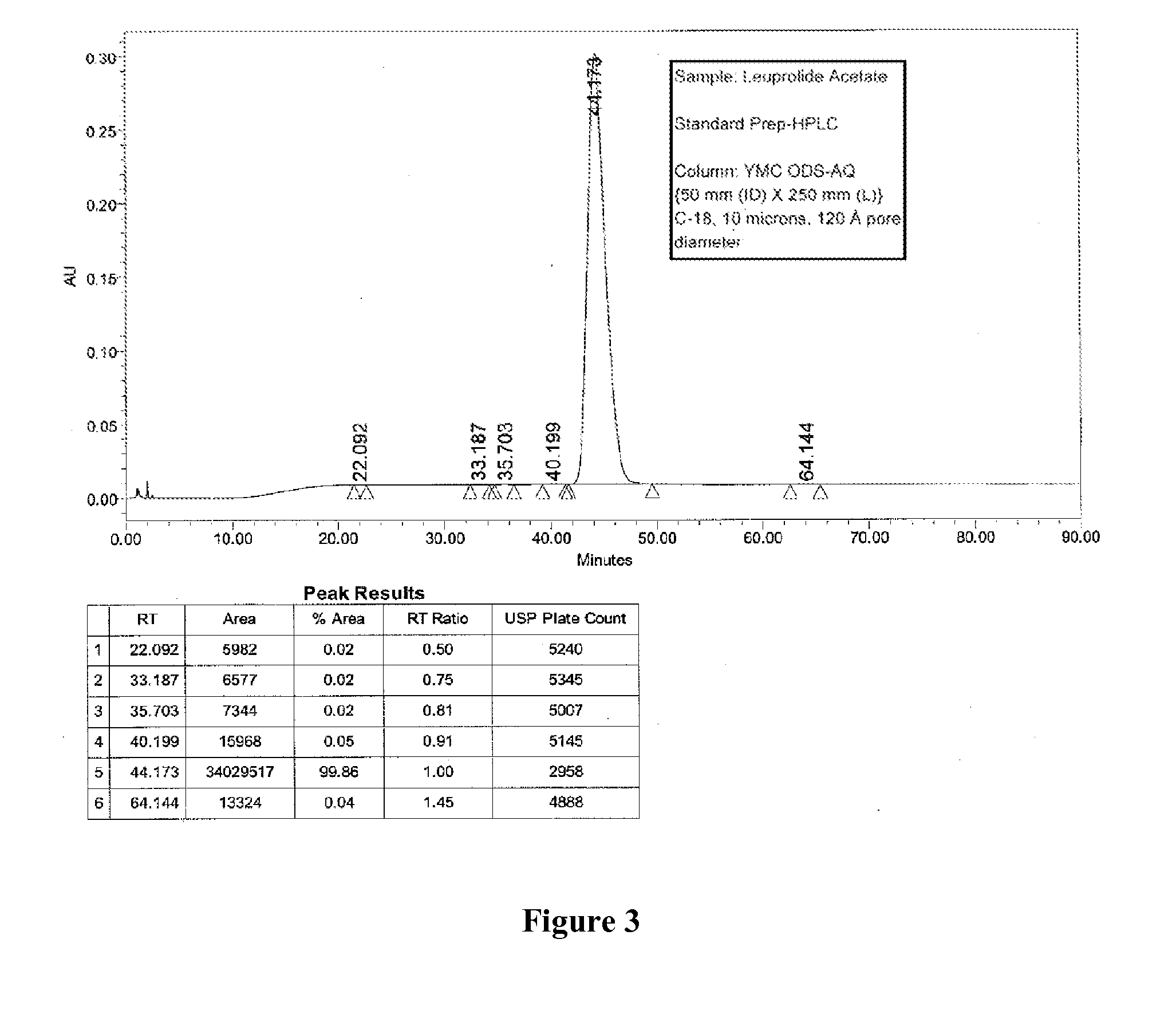
[0090]Two different columns were evaluated for the purification of Leuprolide: A Discovery Bio Wide Pore column {column parameters: 10 mm (ID)×250 mm (L), C18, 5 u particles, 300{acute over (Å)} pore diameter, Amount loaded was 1.2 g of crude Leuprolide (prepared by solution phase synthesis) and a Waters Symmetry Column {column parameters: 19 mm (Internal Diameter, ID)×50 mm (Length, L), C8, 5 u particles, 120 {acute over (Å)}pore diameter, Amount loaded was 1.4 g of crude Leuprolide (prepared by solution phase synthesis) were used. The column was pre-equilibrated with 5 to 10 column volumes (Vcs) of 10 mM TBAHS in 10% aqueous acetonitrile (Buffer A). After the loading was complete, the column was washed with 2 Vcs of Buffer A. Next, the gradient elution process was started. The buffer B was 300 mM TBAHS in 10% aqueous acetonitrile. A linear gradient of 0% B to 100% Buffer B over 60 min. was used for elution. A gradient hold was applied until al
example-2
Preparative RP-HPLC of Triptorelin Acetate
[0092]The C-18 / C-8 reversed phase column was pre-equilibrated with 5 to 10 Vcs of 5 to 10% aqueous acetonitrile containing 10 mM TBAHS (Buffer A). A Discovery Bio Wide Pore column {column parameters: 10 mm (ID)×250 mm (L), C18, 5 u particles, 300{acute over (Å)} pore diameter, Amount loaded was 1.0 g of crude Triptorelin} was used. After the loading was complete, the column was washed with 2 Vcs of Buffer A. Next, the gradient elution process was started. The buffer B was 300 mM TBAHS in 10% aqueous acetonitrile. A linear gradient of 0% B to 100% Buffer B over 60 min. was used for elution. A gradient hold was applied until all the API has eluted from the column. The fractions containing the pure API product were combined and treated with 1.5 to 2 equivalents of sodium tetrafluoroborate (NaBF4) and extracted 3 times with chloroform. The entire purification process was repeated 3 times to demonstrate and confirm the consistent performance. Frac
example-3
Preparative RP-HPLC of Leuprolide Acetate Employing Tetra-n-Butylammonium Bromide (TBA-Br)
[0094]The C-18 reverse phased column [Grace Vydac Column parameters 12 g of C-18, 40 microns particles, 60 Å pore diameter] was saturated with 36 g of TBA-Br in 360 mL of water at the flow rate of 8.0 ml / min. The column was then equilibrated 10 Vcs with Buffer A (25 mM TBA-Br in water) at a flow rate of 8.0 ml / min. he crude leuprolide trifluoroacetate salt was dissolved in Buffer A and loaded on to the column. After the loading is complete, the gradient elution process was started. The Buffer B is 25 mM of TBA-Br in 50% aqueous acetonitrile. A linear gradient of 0% of Buffer B to 100% of Buffer B over 10 Vcs was applied. When Leuprolide is about to elute, a gradient hold may be applied until all the API has eluted from the column. The fraction containing the pure Leuprolide is combined after confirming the purity on an analytical HPLC. Yield: 66.4%. Herein TBA-Br is tetra-n-butylammonium bromi
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap