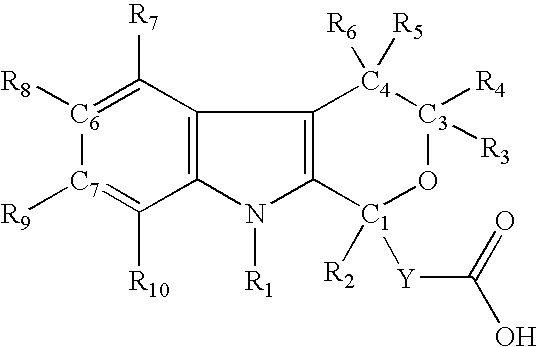
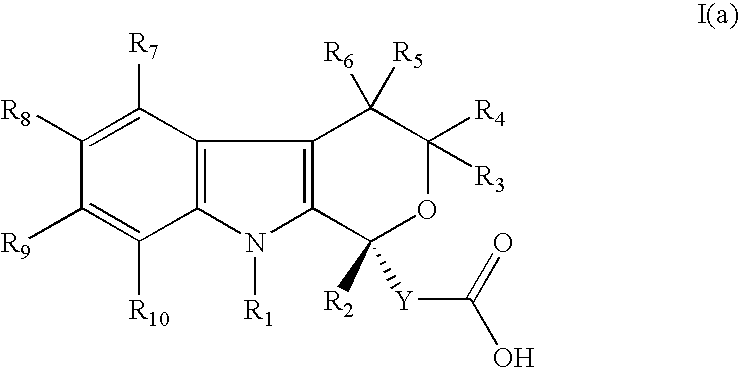
Method for the use of pyranoindole derivatives to treat infection with Hepatitis C virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
EXAMPLE 1
(5-Cyano-8-methyl-1-propyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indol-1-yl)acetic acid
5-Bromo-2-methylaniline
[0268]The mixture of Fe powder (9.31 g, 167 mmol) and NH4Cl (2.48 g, 46.3 mmol) in water (50 mL) was refluxed for 30 minutes. To this hot mixture was added 4-bromo-2-nitrotoluene (10 g, 46.3 mmol) slowly and then the reaction mixture was refluxed for 48 hours. The mixture was cooled to room temperature and extracted with EtOAc (3×100 mL). The organic solution was washed with H2O (3×200 mL) and brine (200 mL), dried (Na2SO4), and concentrated. The residue was purified by flash chromatography (silica, 15% EtOAc in hexanes) to give 7.9 g (92%) of title compound as a pale yellow oil. 1H nuclear magnetic resonance (NMR) (CDCl3): 300 MHz δ 6.88 (m, 1H), 6.81 (m, 2H), 3.63 (bs, 2H), 2.09 (s, 3H).
5-Bromo-2-methylphenylhydrazine Hydrochloride
[0269]To a suspension of 5-bromo-2-methylaniline (4.80 g, 25.8 mmol) in concentrated HCl (16 mL) was added dropwise a solution of sodium nitrit
Example
EXAMPLE 2 AND EXAMPLE 3
[(R)-5-cyano-8-methyl-1-propyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl]acetic acid
[(S)-5-cyano-8-methyl-1-propyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl]acetic acid
Resolution of (±)-5-Cyano-8-methyl-1-propyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic acid
[0274]Preparative high pressure liquid chromatography (HPLC) using CHIRALPACK-AD (250×20 mm) and 10% isopropyl alcohol in heptane (0.1% trifluoroacetic acid (TFA)) as eluant gave (R) and (S) enantiomers of 5-cyano-8-methyl-1-propyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic acid as white solids. HRMS (ESI) [M+H]+ calculated for C18H21N2O3 313.1547, found 313.1545 (R enantiomer) and 313.1547 (S enantiomer); Chiral HPLC HP 1100 with spiderlink CHIRALPACK-AD, 250×4.6 mm, isopropyl alcohol / heptane containing 0.1% TFA (10:90), 1.0 mL / minutes, DAD 215 nm; tR=6.98 minutes (R enantiomer), 9.37 minutes (S enantiomer).
[0275]Alternatively, [(R)-5-cyano-8-methyl-1-propyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-
Example
[0276]The absolute configuration of the compound of Example 2 was determined by single crystal X-ray crystallography of the 4-bromobenzyl amide derivative, which was prepared as described below.
1-(R)-N-(4-Bromo-benzyl)-2-(5-cyano-8-methyl-1-propyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-acetamide
[0277]To a solution of 1-(R)-5-cyano-8-methyl-1-propyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic acid (20.0 mg, 0.064 mmol), 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (EDCI, 15.0 mg, 0.077 mmol) and 1-hydroxybenzotriazole (10.4 mg, 0.077 mmol) in DMF (4 mL) was added N,N-diisopropylethylamine (67 μl, 0.384 mmol) followed by 4-bromobenzylamine hydrochloride (17.1 mg, 0.077 mmol) at room temperature. The reaction mixture was stirred for 20 hours at ambient temperature. Water (5 mL) was added to the mixture and the resulting mixture was extracted with EtOAc (3×10 mL). The combined organic phase was washed with brine (20 mL), dried over Na2SO4 and concentrated. The resi
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap