Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4 results about "Highly sensitive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for solid-phase enrichment and mass spectrographic analysis of glycosylated peptide fragment
ActiveCN105300783AImprove Analytical Mass Spectrometry SelectivityHigh reaction specificityPreparing sample for investigationMaterial analysis by electric/magnetic meansGlycopeptideMass spectrometric
Owner:FUDAN UNIV
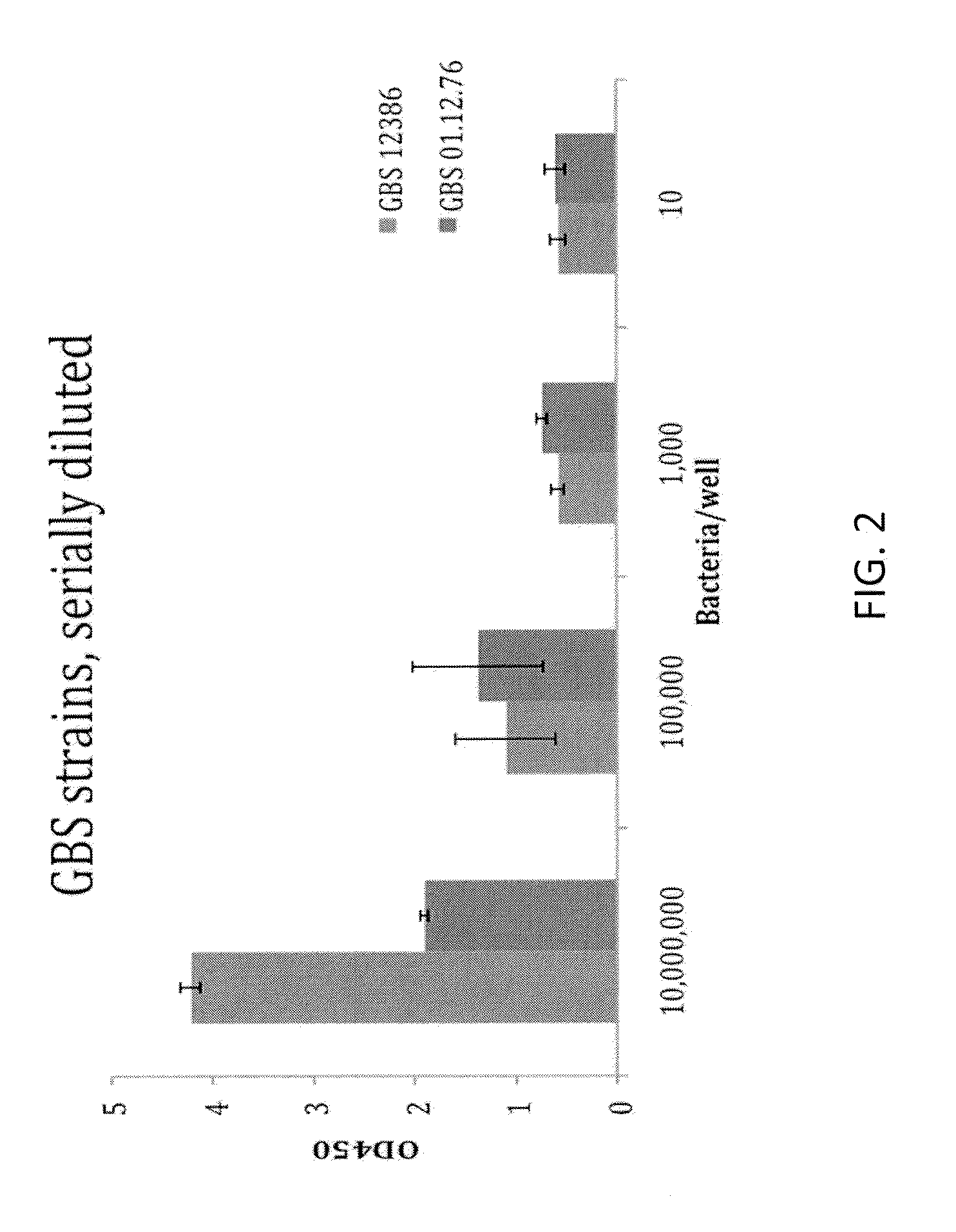
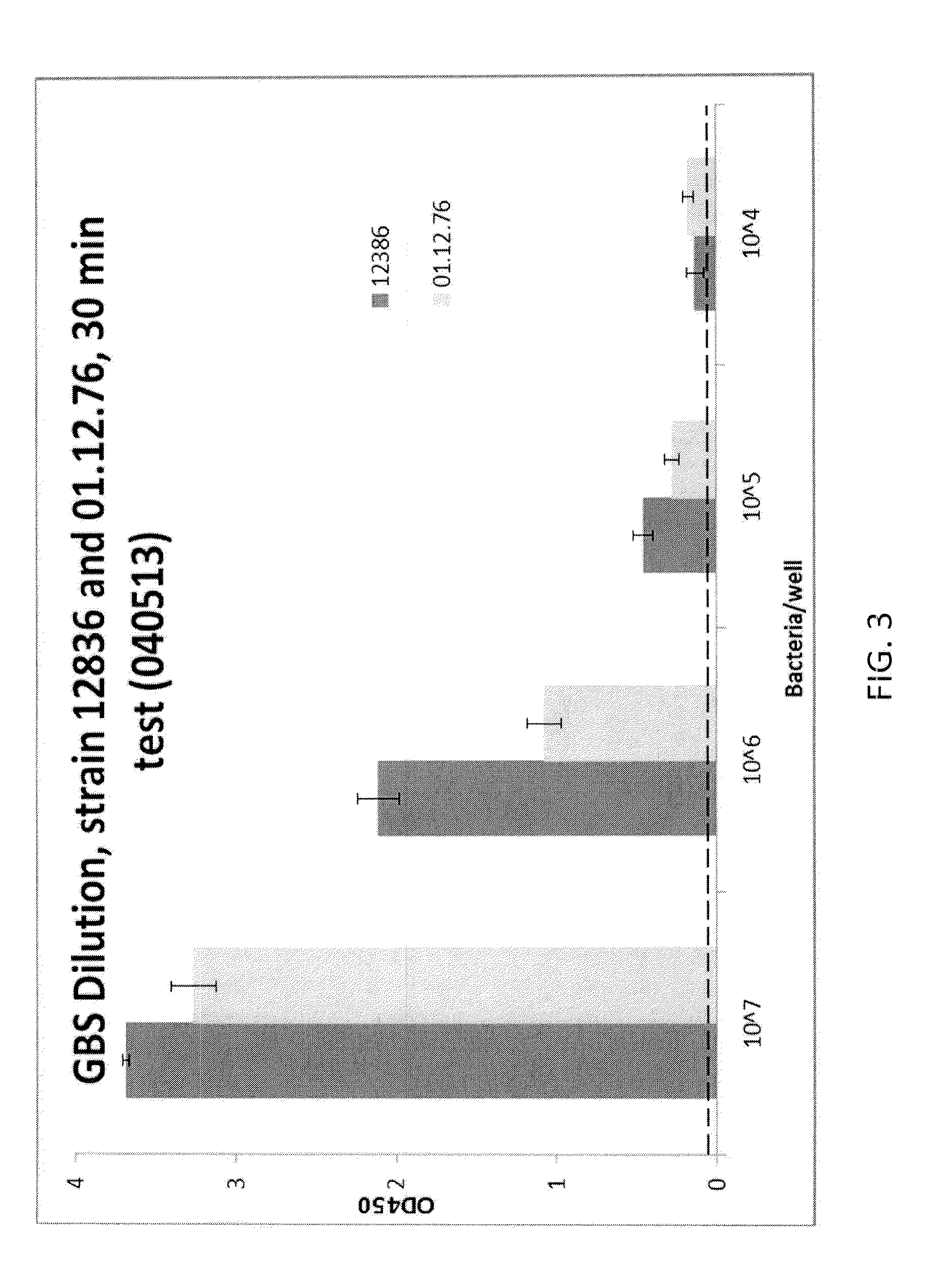
Rapid Enzyme-Linked Immunosorbant Assay for Detection and Identification of Pathogens and Determination of Antimicrobial Susceptibility
ActiveUS20140315219A1High sensitivityStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsNon targetedNon target
Owner:BARNHIZER BRET T +1
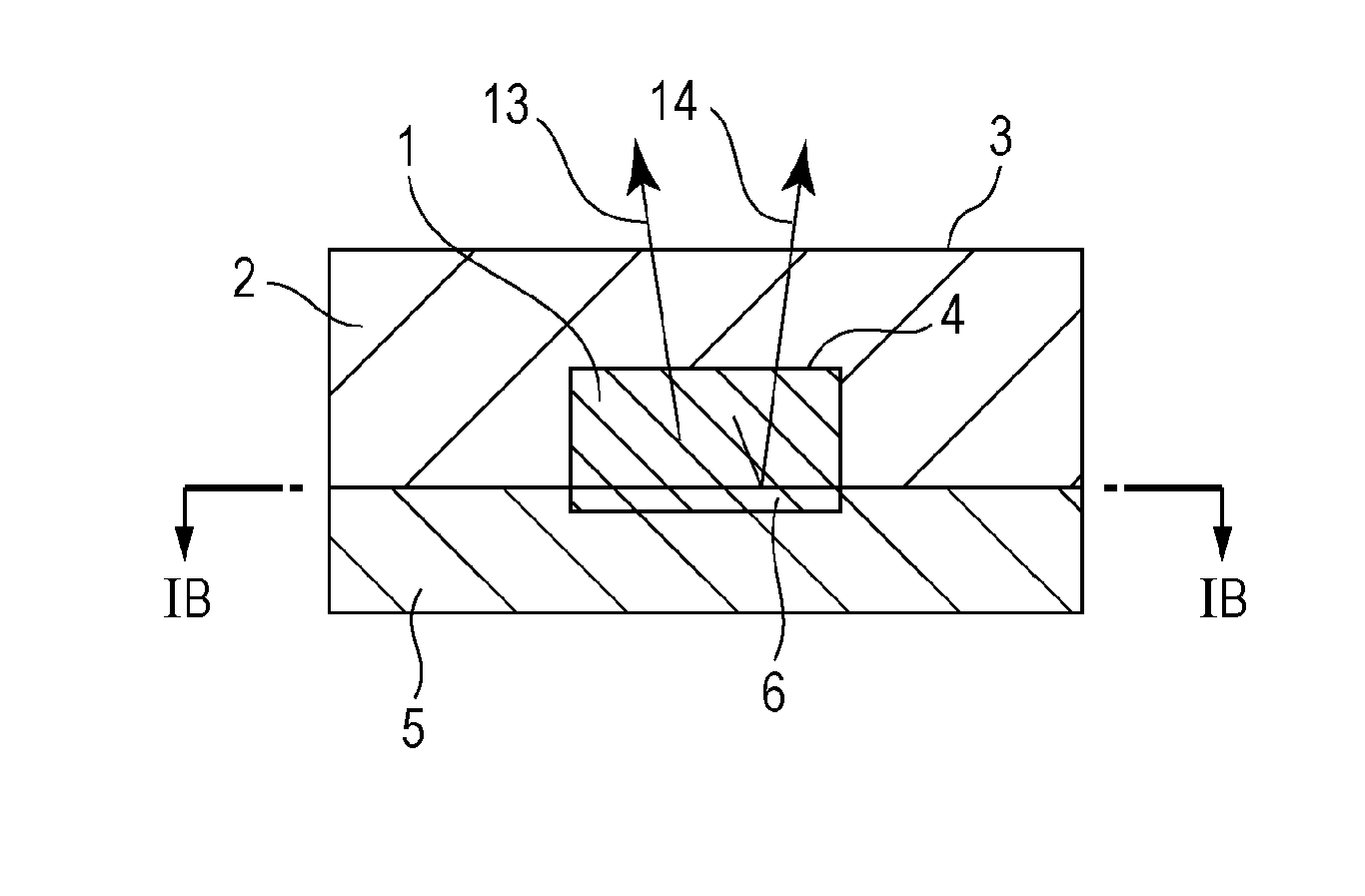
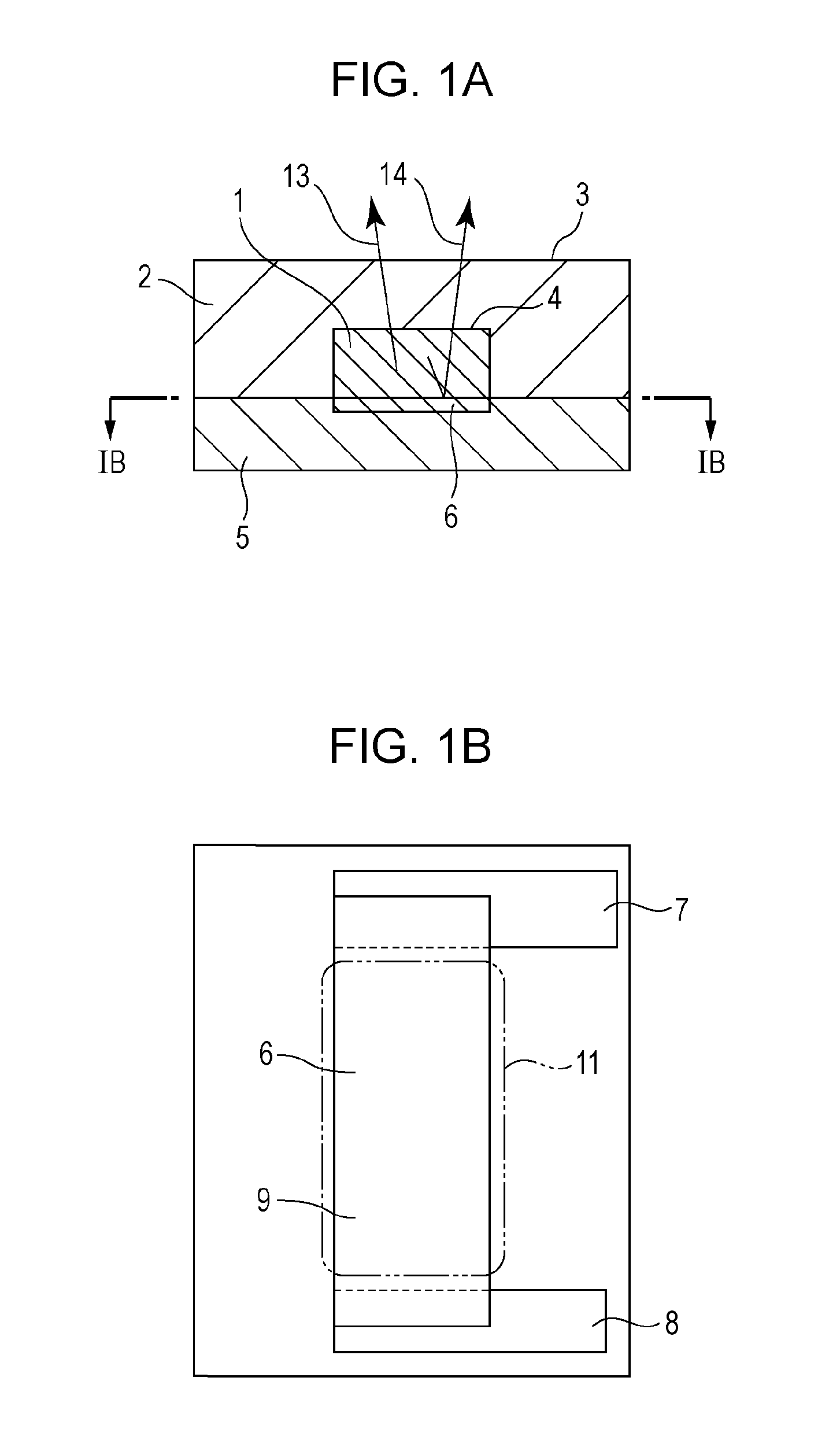
Flow passage device and testing system using the same
ActiveUS20140106984A1Highly sensitive real time analysisSimple structureBioreactor/fermenter combinationsBiological substance pretreatmentsElectrical resistance and conductanceReal time analysis
Owner:CANON KK
MULTIPLEX ASSAY FOR DETERMINING beta-AMYLOID 42/40 RATIO IN HUMAN PLASMA SPECIMENS
The present technology relates to methods for diagnosing, monitoring the progression of, assessing the efficacy of treatment of, or assessing risk for development of a neurodegenerative disorder in a patient. These methods are based on determining the ratio of beta-amyloid 42 ("A beta42") to beta- amyloid 40 ("A beta40") in a body fluid sample collected from a patient who has or is suspected of having a neurodegenerative disorder, using an improved and highly sensitive multiplex protein assay that simultaneously detects A beta42 and A beta40.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap