Lithium ion secondary battery positive electrode active material, and production method thereof
A cathode active material, secondary battery technology, applied in battery electrodes, lithium storage batteries, non-aqueous electrolyte storage batteries, etc., can solve the problems of the cathode active material easily agglomerated coarse particles, a large amount of thermal energy, etc., to achieve excellent cycle characteristics and rate characteristics. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0217] 3.0 g of distilled water was added to 7.0 g of an aluminum lactate aqueous solution having an aluminum content of 4.5% by mass and a pH of 4.6, and mixed to prepare an aluminum lactate aqueous solution. In addition, in ammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) to 0.77 g of distilled water 9.23 g was added and mixed to prepare an ammonium hydrogen phosphate aqueous solution.
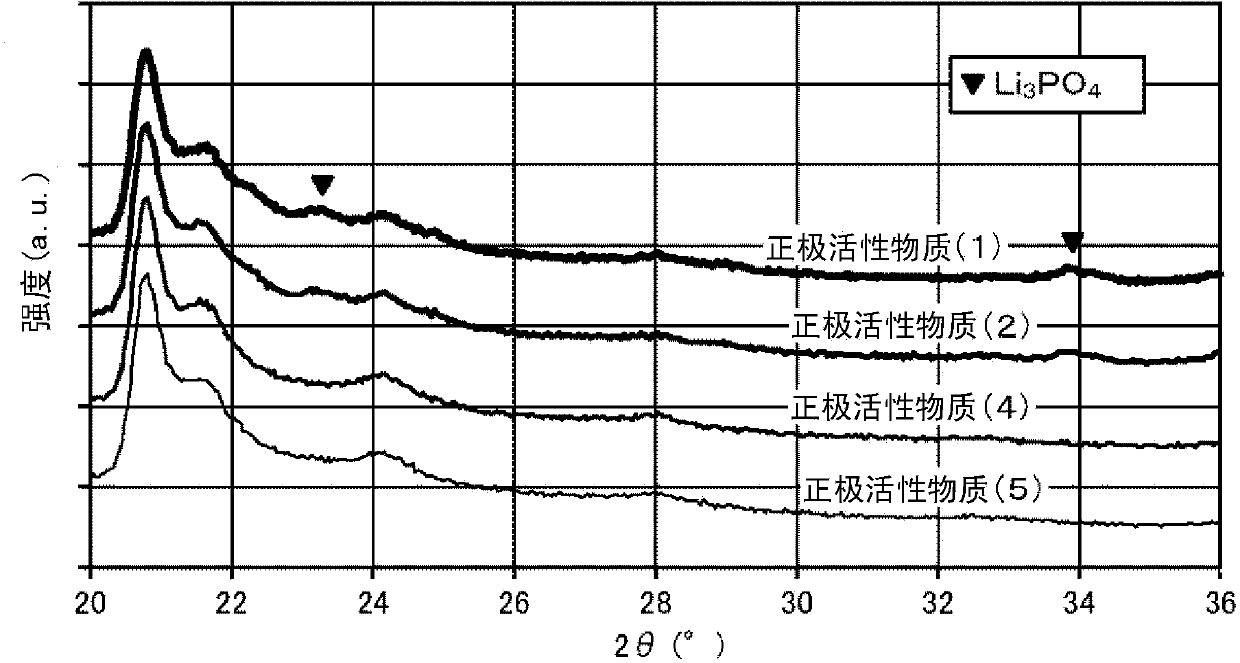
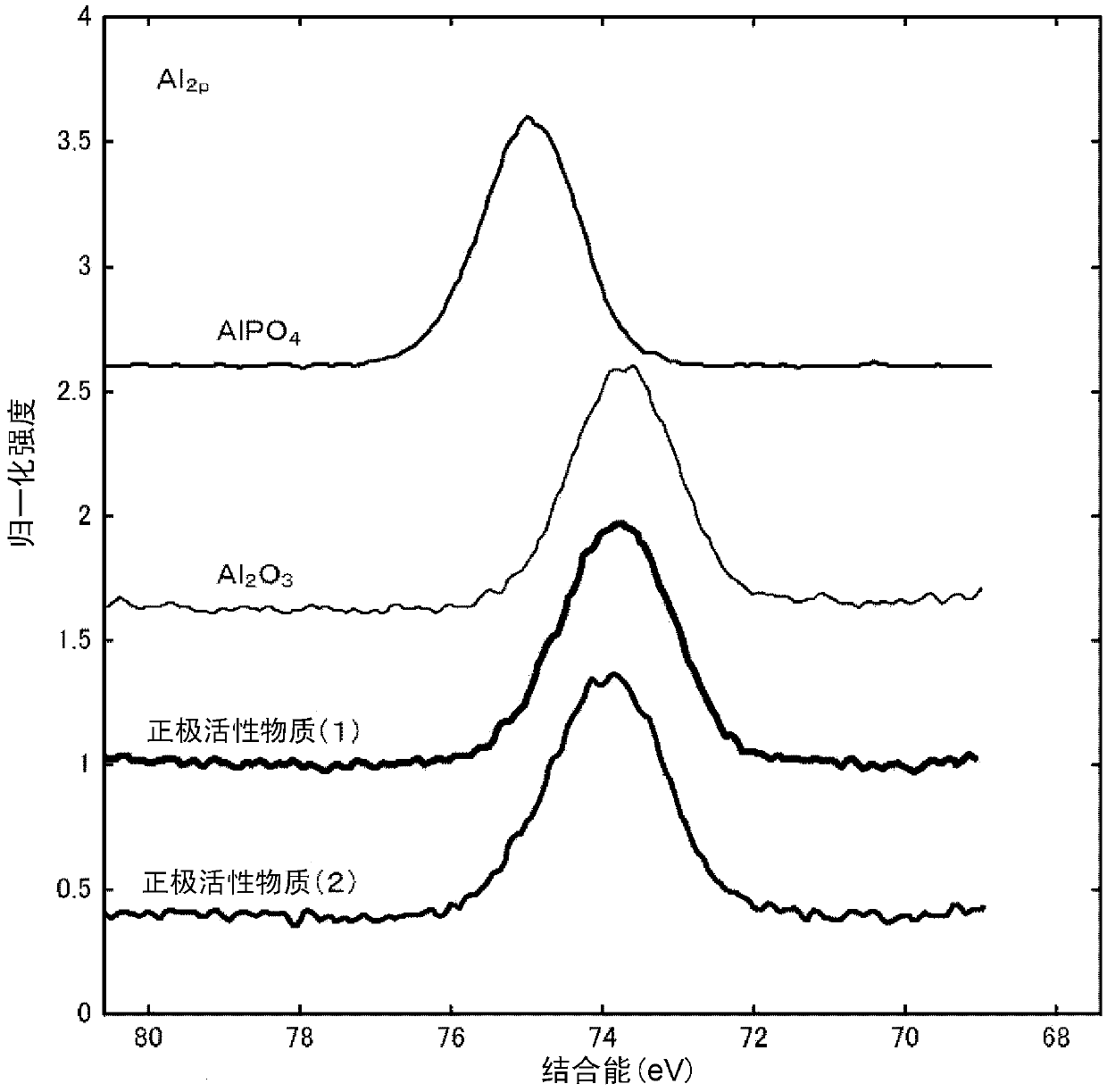
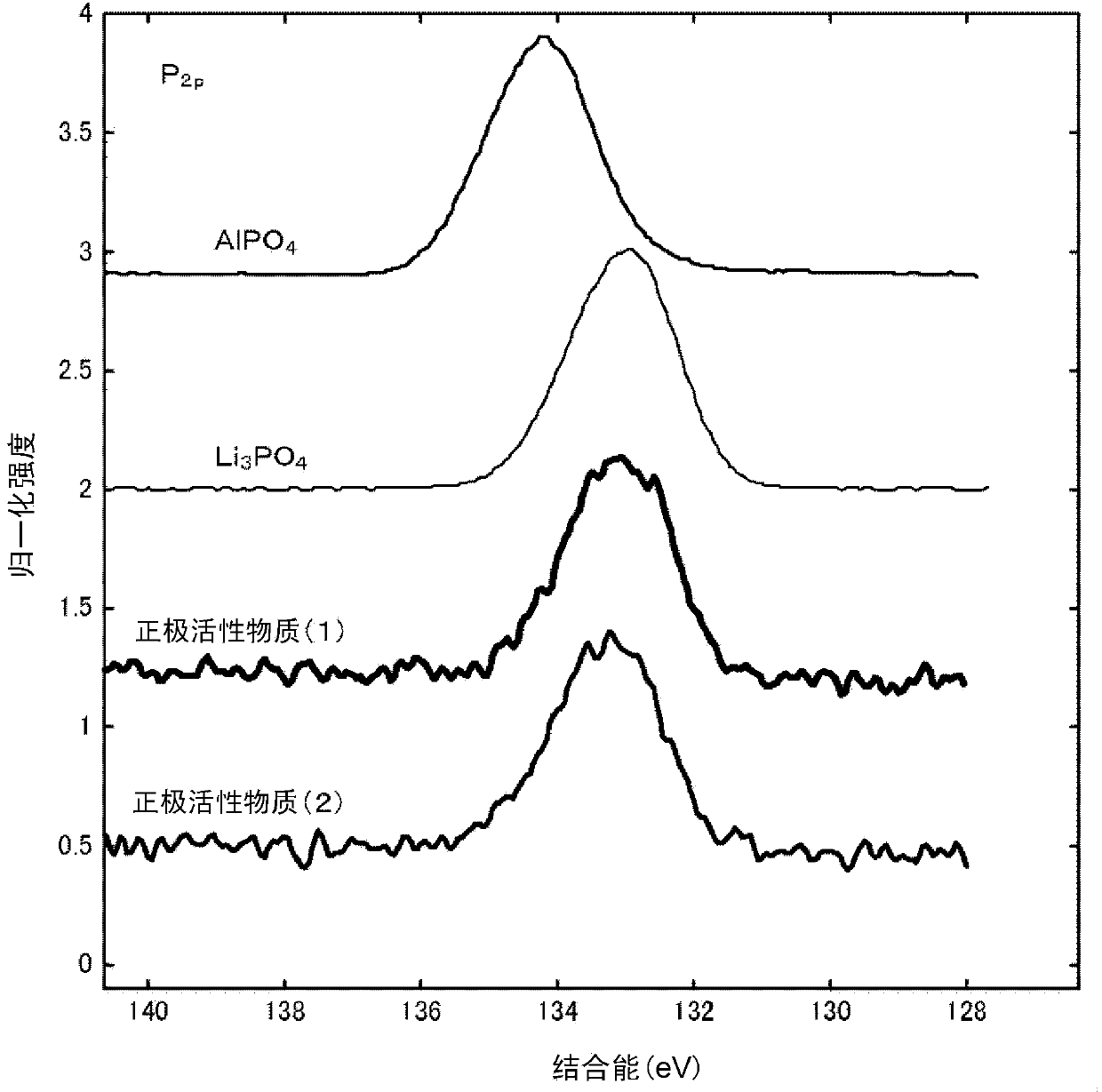
[0218] Next, while stirring the lithium-containing composite oxide (A) obtained as described above, 1 g of the above-prepared ammonium hydrogen phosphate aqueous solution was sprayed on 10 g of the lithium-containing composite oxide (A) by a spraying method to make the lithium-containing composite oxide ( A) It is brought into contact with the ammonium hydrogen phosphate aqueous solution while being mixed. Next, 1 g of the aluminum lactate aqueous solution prepared above was sprayed by a spray coating method, and the lithium-containing composite oxide (A) and the aluminum lactate aqueous solut
Embodiment 2
[0226] In ammonium hydrogen phosphate ((NH 4 ) 2 HPO 4) was added to 1.23 g of distilled water to prepare an ammonium hydrogen phosphate aqueous solution. Next, except that the ammonium hydrogen phosphate aqueous solution was sprayed on the lithium-containing composite oxide (A) to make it contact, the same operation was performed as in Example 1 to obtain a positive electrode active material (2). The surface of the lithium composite oxide particles is composed of particles (III) having a coating layer containing Al and P.
[0227] In addition, the cation (Al 3+ ) and anion (PO 4 3- ) has a value of (Z) of 0.80.
[0228] In the positive electrode active material (2) obtained as described above, the value of the molar ratio of Al contained in the coating layer to the lithium-containing composite oxide by the aluminum lactate aqueous solution is determined by {(Al in the coating layer (I) The number of moles) / (the number of moles of the lithium-containing composite oxide)}
Embodiment 3
[0232] In ammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was added to 0.46 g of distilled water to prepare an ammonium hydrogen phosphate aqueous solution. Next, except that the ammonium hydrogen phosphate aqueous solution was sprayed on the lithium-containing composite oxide (A) to make it contact, the same operation was performed as in Example 1 to obtain a positive electrode active material (3). The positive electrode active material (3) was obtained by containing The surface of the lithium composite oxide particles is composed of particles (III) having a coating layer containing Al and P.
[0233] In addition, the cation (Al 3+ ) and anion (PO 4 3- ) has a value of (Z) of 0.30.
[0234] In the positive electrode active material (3) obtained as described above, the value of the molar ratio of Al contained in the coating layer to the lithium-containing composite oxide through the aluminum lactate aqueous solution is determined by {(Al in the coating layer (I) The number
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap