Strontium-free and cobalt-free perovskite type composite oxide and preparation method thereof, and battery
A composite oxide and perovskite-type technology, applied in fuel cells, battery electrodes, solid electrolyte fuel cells, etc., can solve the problems of high battery overpotential, insufficient performance at medium temperature, Cr poisoning, etc., to improve performance , Improve long-term stability and lack of performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The embodiment of the present invention also provides a method for preparing the above-mentioned strontium-free and cobalt-free perovskite composite oxide. The embodiment of the present invention prepares the strontium-free and cobalt-free perovskite composite oxide by using the sol-gel method. things. Specifically, including:
[0037] According to different components La 1-x Ni 0.6 Fe 0.4 o 3 (0≤x<0.1, such as x=0, 0.02, 0.04, 0.06 and 0.08) each metal element metering ratio accurately weighs each metal salt, and then mixes and dissolves the above-mentioned metal salts in water to form a metal salt mixed solution, so that The molar ratio of La, Ni and Fe in the metal salt mixed solution is 1-x:0.6:0.4, 0≤x<0.1.
[0038] Wherein, the metal salt can be any one of nitrates, carbonates and acetates, and the metal salt mixed solution formed is also any one of the above-mentioned nitrates, carbonates and acetates.
[0039] Then EDTA and ammonia water are mixed and dissolv
Embodiment 1
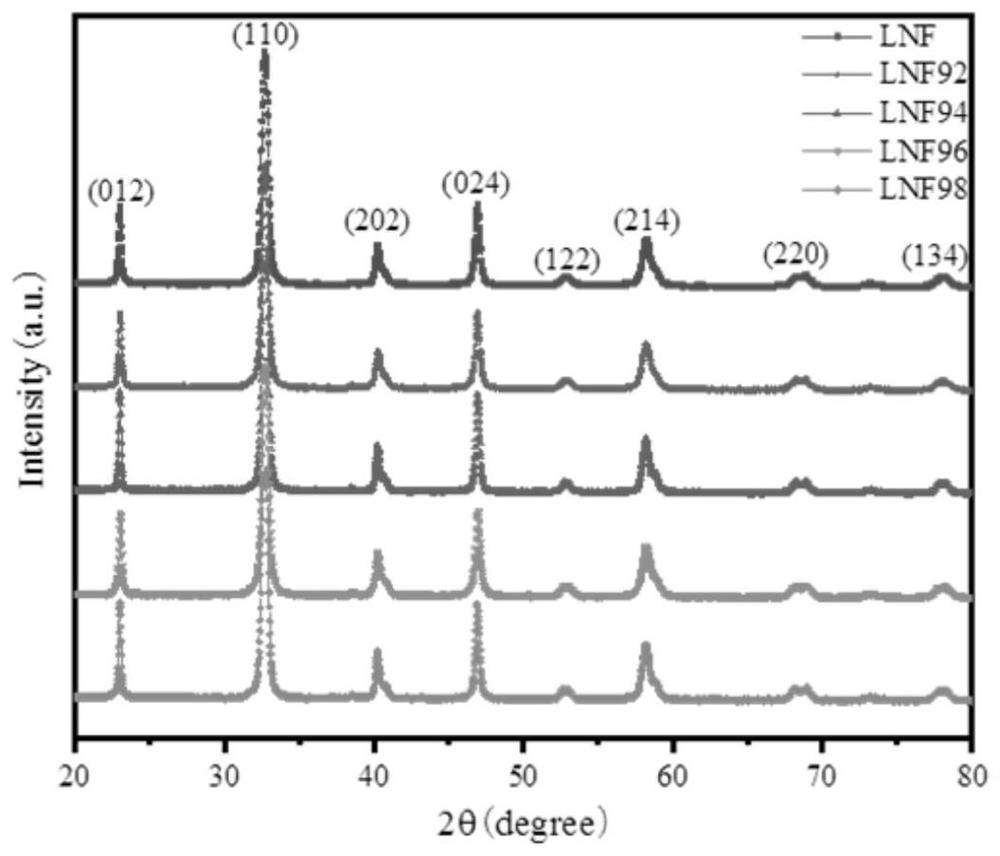
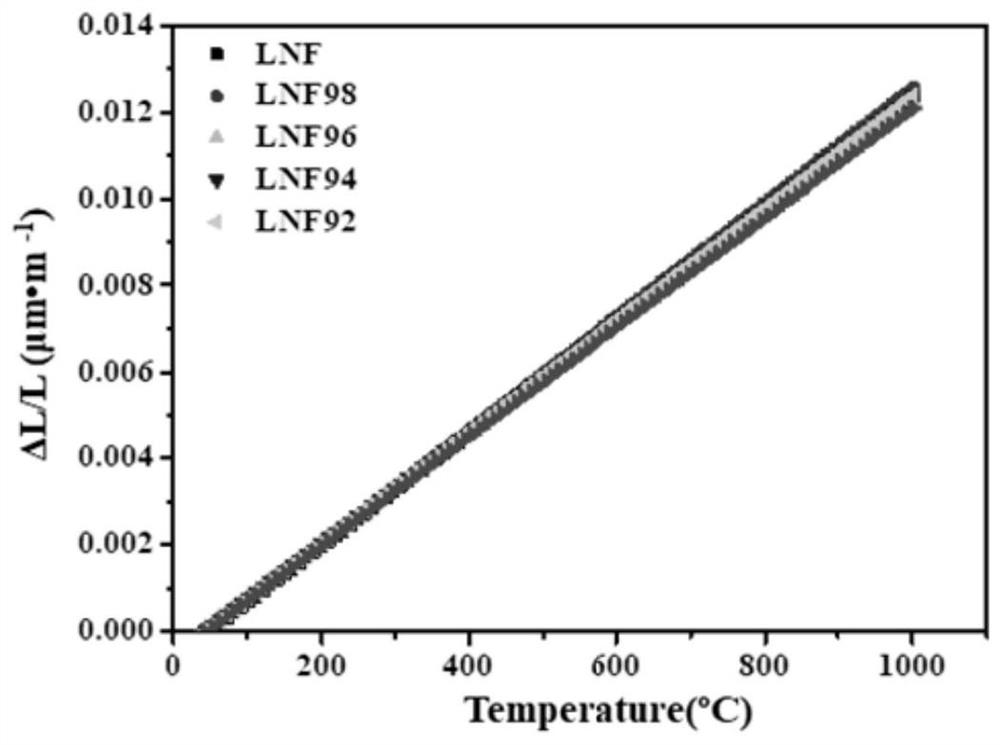
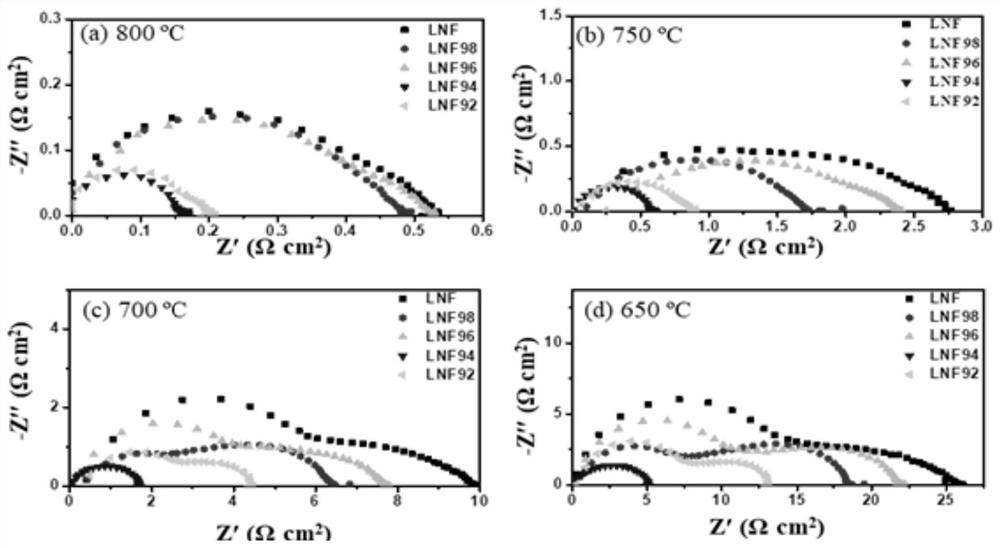
[0050] The embodiment of the present invention provides a strontium-free and cobalt-free perovskite-type composite oxide whose chemical formula is LaNi 0.6 Fe 0.4 o 3 (Number: LNF).
[0051] The embodiment of the present invention also provides a method for preparing the above strontium-free and cobalt-free perovskite-type composite oxide, including:
[0052] Accurately weigh the nitrates containing La, Ni and Fe respectively, and the molar ratio of La, Ni and Fe is 1:0.6:0.4. Accurately weigh EDTA and glycine, and the sum of metal cations: EDTA: glycine = 1:1:1.5 (molar ratio).
[0053] Then dissolve the above three nitrates in a 1000ml large beaker with clear deionized water. After about 15 minutes, the metal nitrates can be completely dissolved. Place the beaker in a constant temperature oil bath and stir at a constant temperature of 80°C at a stirring speed of 400r / min. Metal salt mixed solution.
[0054] Then dissolve EDTA in a 100ml small beaker with ammonia water an
Embodiment 2- Embodiment 5
[0060] Embodiment 2 provides a strontium-free and cobalt-free perovskite composite oxide, whose chemical formula is La 0.98 Ni 0.6 Fe 0.4 o 3 (No.: LNF98).
[0061] Embodiment 3 provides a strontium-free and cobalt-free perovskite composite oxide, whose chemical formula is La 0.96 Ni 0.6 Fe 0.4 o 3 (No.: LNF96).
[0062] Embodiment 4 provides a strontium-free and cobalt-free perovskite composite oxide, whose chemical formula is La 0.94 Ni 0.6 Fe 0.4 o 3 (No.: LNF94).
[0063] Embodiment 5 provides a strontium-free and cobalt-free perovskite composite oxide, whose chemical formula is La 0.92 Ni 0.6 Fe 0.4 o 3 (No.: LNF92).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal expansion coefficient | aaaaa | aaaaa |
| Thermal expansion coefficient | aaaaa | aaaaa |
| Thermal expansion coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap