Sustained-release capsule containing tacrolimus solid dispersion and preparation method thereof
A technology of solid dispersion and tacrolimus, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, capsule delivery, etc., can solve problems that are not conducive to industrialization and increase production costs, and achieve The effect of reducing the number of doses, easy to reproduce, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040]
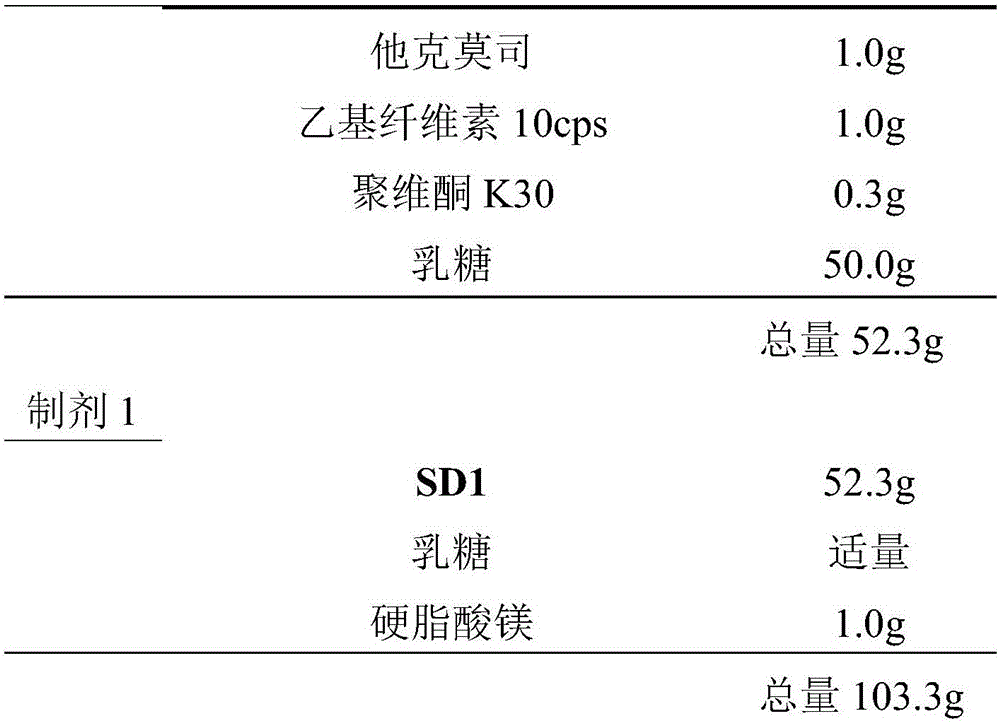
[0041] Dissolve tacrolimus in ethanol, add ethyl cellulose and stir to dissolve it, slowly add povidone K30 to the above solution, and keep stirring until it is fully dissolved. Add the crushed and 200-mesh lactose into the wet granulation pot, pre-mix for 5 minutes, then slowly add the above solution to granulate through the wet granulator equipment. After granulation, put it on a tray, dry it in a common oven at 40°C, and then sieve it through a 70-mesh sieve to obtain SD1. Add lactose, SD1 and magnesium stearate to the mixer in sequence, and mix well to obtain Preparation 1. Formulation 1 was filled in capsules.
Embodiment 2
[0043]
[0044]
[0045] Formulation 2 was prepared using the same method as described in Example 1. Formulation 2 was filled in capsules.
Embodiment 3
[0047]
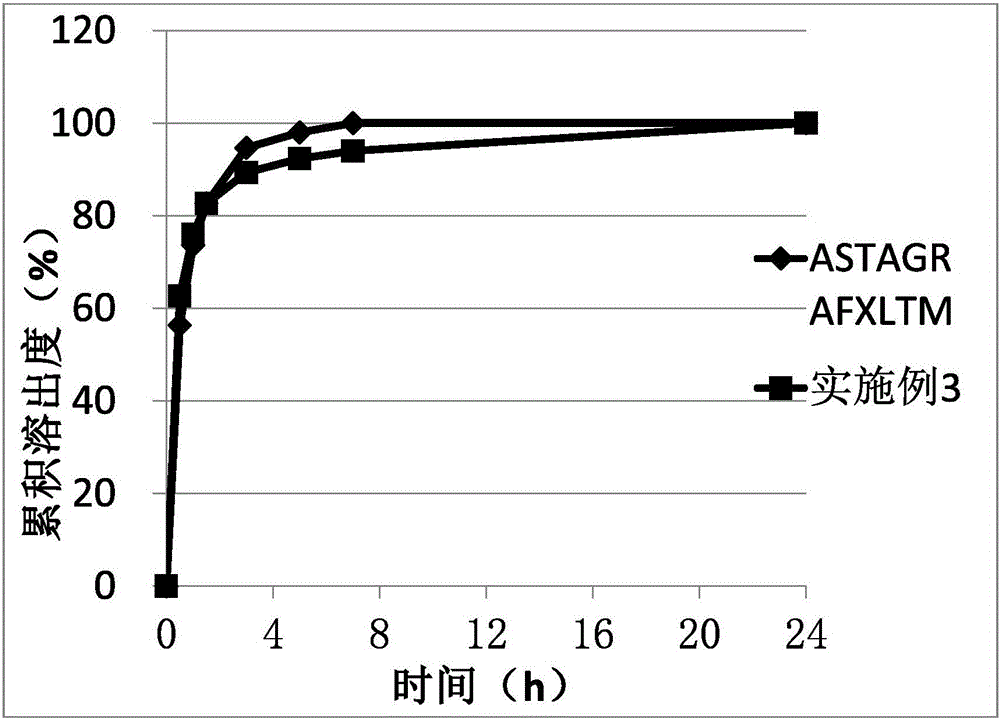
[0048] Formulation 3 was prepared using the same method as described in Example 1. Formulation 3 was filled in capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap